Abstract
Context
An increased risk of depressive symptoms has been associated with the transition to menopause, but the risk of depressive symptoms in the early postmenopausal years has not been well-characterized.
Objectives
Identify within-woman changes in depressive symptoms over a 14-year period around menopause, determine associations of a history of depression with the pattern of depressive symptoms and evaluate the rate of change in reproductive hormones as predictors of depressive symptoms postmenopause.
Design
A cohort of late reproductive-age women followed for 14 years with annual assessments of symptoms and reproductive hormones.
Setting
Randomly-identified, population-based sample in Philadelphia County, PA
Participants
203 generally healthy women who were premenopausal at baseline and reached natural menopause in the follow-up interval.
Main Outcome Measure
Center for Epidemiologic Studies Depression Scale (CES-D)
Results
The prevalence of high CES-D scores decreased from 10 years before menopause to 8 years postmenopause, with a decrease of approximately 15% of baseline per year (odds ratio 0.85, 95% CI: 0.81–0.89, P<0.001). Relative to the final menstrual period (FMP), the risk of depressive symptoms was greater in the years before and lower in the years following the FMP. Among women with a history of depression, the likelihood of depressive symptoms was 13 times greater overall and 8 times greater postmenopause compared to women with no depression history. Among women who first experienced depressive symptoms approaching menopause, the risk of depressive symptoms declined after the FMP, with a significantly lower risk after the second year postmenopause. The risk of depressive symptoms postmenopause decreased by 35% for each unit (SD) increase before the FMP in the log rate of change of follicle stimulating hormone (FSH) (odds ratio 0.65, 95% CI 0.46–0.91, P=0.01).
Conclusions
The final menstrual period was pivotal in the overall pattern of decreasing depressive symptoms in mid-life women, with greater risk before and lower risk following the FMP. A history of depression strongly increased the risk both before and after menopause. Women who had no history of depression before the menopause transition had a low risk of depressive symptoms after two or more years following the FMP.
INTRODUCTION
Multiple studies show an increased risk of depression in the years leading to menopause, with some evidence that changes in reproductive hormones contribute to these symptoms in vulnerable women.1–7 Whether this same risk continues in the early postmenopausal years has not been well-characterized. Information about the risk of depression in mid-life women is clinically important due to the diminished functioning and significant disability that accompany this common disorder,8 and because depression is associated with other health-limiting conditions that increase in mid-life women, such as cardiovascular disease, metabolic syndrome and osteoporosis.9–12
The increased risk of depressive symptoms in the transition to menopause has been repeatedly observed in population-based studies. In the Penn Ovarian Aging Study (POAS), the risk of depressive symptoms was nearly three times greater in the menopause transition compared to premenopausal women, 1 while women who had no previous history of depression were 2 ½ times more likely to report depressed mood in the menopause transition compared to when they were premenopausal.3 Other cohort studies reported similar findings: the Harvard Study of Moods and Cycles,2 the Study of Women Across the Nation (SWAN), 13 the Seattle Midlife Women’s Health Study (SMWHS), 14 and the Melbourne Women’s Midlife Health Project.15
In contrast to these studies of the years approaching menopause, there is limited information on the risk of depression in the years following menopause. In one SWAN study, depressive symptoms improved after the final menstrual period,16 but in another study that evaluated major depression, the risk of major depression remained high through early postmenopause compared to premenopause.13 While national survey data indicate that the prevalence of depressive disorders is highest in women ages 40–49 years and lowest in women older than 60 years, 17 the risk of depressive symptoms in the decade between these years has not been identified.
The present study was conducted to identify within-woman changes in depressive symptoms over a 14-year period around natural menopause. We hypothesized that relative to menopause, the risk of high depressive symptoms was greater in the years preceding menopause and decreased in the years following menopause. Additional objectives were to determine the association of a history of depression with the pattern of depressive symptoms, evaluate other covariates of age at menopause, race, body mass index, current smoking as possible modifiers of the depressive symptoms, and identify the within-woman rate of change of reproductive hormones (estradiol, follicle stimulating hormone [FSH] and inhibin b) in the years leading to menopause as predictors of depressive symptoms in the early postmenopausal years.
MATERIALS AND METHODS
Study participants
The study evaluated 203 premenopausal women in the Penn Ovarian Aging Study (POAS) who reached natural menopause during a 14-year follow-up period (1996–2010). The POAS cohort was randomly identified by telephone digit dialing to households in Philadelphia County, PA, and sampling was stratified to obtain equal numbers of African American and white women as described in previous reports.18 All cohort participants were premenopausal at enrollment as defined by regular menstrual cycles in the reference range (22–35 days for the previous three menstrual cycles), ages 35–48 years, and had an intact uterus and at least one ovary. Exclusion criteria included current use of any hormone or psychotropic medication, alcohol or drug abuse, major psychiatric disorder in the past year, pregnancy or breast feeding, serious health problems known to compromise ovarian function or uncontrolled hypertension.
Comparisons of the study variables at baseline between the study sample (N=203) and the remainder of the cohort (N=233 who were excluded because they had not reached menopause in the follow-up interval) showed that study participants were slightly older, had higher FSH levels and lower inhibin b levels compared to the remainder of the cohort; other variables in Table 1 did not significantly differ between the two groups. The Institutional Review Board of the University of Pennsylvania approved the study, and all participants provided written informed consent.
Table 1.
Study variables at baseline
| Variable | Baseline (n=203) |
|---|---|
| Age, mean (SD), y | 42.77 (3.12) |
| Age at FMP, mean (SD), y | 51.10 (3.30) |
| BMI, kg/m2, mean (SD) | 29.22 (7.68) |
| CES-D ≥ 16, n(%) | 81 (40) |
| CES-D ≥ 25, n(%) | 35 (17) |
| Estradiol, pg/mL, mean (95% CI) | 33.38 (30.34 – 36.71) |
| FSH, mIu/mL, mean (95% CI) | 7.38 (6.97 – 7.82) |
| Inhibin b, ng/mL, mean (95% CI) | 55.98 (50.97 – 61.48) |
| Current smoker, n(%) | 75 (37) |
| History of depression, n(%) | 90 (44) |
| Race, n(%) | |
| African American | 96 (47) |
| White | 107 (53) |
Study design
Following cohort enrollment, follow-up assessments were conducted for 14 years at intervals of approximately 9 months in the first five years and then annually, with a two-year gap between assessments 10 and 11. At each assessment period, study data were collected at two in-home visits timed to the early follicular phase of the menstrual cycle (days 2–6), in two consecutive menstrual cycles or approximately one month apart in non-cycling women.
The study was described to participants as a general women’s health study. Trained research interviewers obtained menstrual dates, structured interview data on overall health, blood samples for the hormone assays, and anthropometric measures. Participants completed a set of validated self-report measures to assess health and other behavioral measures of the study at each assessment period.
Study variables
The primary outcome variable of depressed mood was assessed by the Center for Epidemiologic Studies Depression Scale (CES-D),19 a standard measure that assesses current depressive symptoms in the past week. Participants rated the 20 items on a 4-point scale (0–3) at each assessment period. The standard cut point of >=16 in the total score defined the high depressive symptom group. A higher cut point score of >=25, which has greater specificity for a clinical diagnosis of depression20, 21 was also examined. In the postmenopausal predictive models, depression status was categorized in four groups: (1) history of depression as identified at enrollment, (2) depressive symptoms first occurred during the study while premenopausal or (3) in the menopause transition, and (4) no depression or depression history before the final menstrual period. Time in years, from 12 years before to 11 years after the FMP, was evaluated in relation to the final menstrual period (FMP), which was identified after 12 or more months of no menstrual bleeding and designated as Time 0 for each subject to allowlongitudinal evaluation of within-woman changes in depressive symptoms each year before and after menopause.
Covariate selections were based on previously identified associations with depressed mood: history of depression (yes, no) as identified at cohort enrollment by medical history interview or the Primary Care Evaluation of Mental Disorders (PRIME-MD) interview;22 race (self-reported as African American or white); and age, body mass index (BMI: kilograms per meter squared), and current smoking (yes, no) at the FMP. Menopausal stages were based on the Staging System for Reproductive Aging in Women (STRAW)23 as follows: premenopausal: regular cycles in normal range (22–35 days); a combined menopause transition group: cycle length changes >=7 days either direction relative to the subject’s own baseline through 11 months of amenorrhea; postmenopause: identified after 12 or more months of no menstrual bleeding. Current medication (yes, no) included antidepressant and anxiolytic medications reported at follow-up assessments. These medications were exclusions at cohort enrollment. At each follow-up period, 2 to 15 participants reported medication use, for a total of 147 (6.13%) of the observations. Of these, there were 50 (7.5%) occurrences of medication use among 30 participants after FMP.
Non-fasting blood samples were collected at each study visit between days 2 and 6 of the menstrual cycle in two consecutive cycles (or approximately one month apart in non-cycling women) in the 14 assessment periods. The blood samples were centrifuged and frozen in aliquots at −80° C. Assays were performed in the Clinical and Translational Research Center at the University of Pennsylvania and were run in batches that included 4 visits per participant to reduce within-subject variability due to assay conditions. Assays were performed in duplicate and repeated if values differed by more than 15%. Estradiol and FSH were measured by radioimmunoassay using Coat-a-Count commercial kits (Siemens, Deerfield, IL). The intra- and interassay coefficients of variation were less the 5%. For assessment periods 1114, inhibin b was assayed using inhibin B enzyme-linked immunosorbent assay kits (Beckman Coulter, Inc, Brea, CA). The intra-and interassay CVs were 3.5% to 4.6% and 6.3% to 7.6%, respectively. The lower limit of detection was 7 pg/mL. Dimeric inhibin b assays for Assessments 1–10 were conducted in the laboratory of Patrick Sluss, PhD, at the Massachusetts General Hospital (Boston, MA), using a solid-phase sandwich ELISA with plates coated with a monoclonal antibody specific for the alpha subunit of detection. The assay was controlled in triplicate using samples with mean concentrations of 155.3, 316.3, and 919.3 pg/mL with interassay CVs of 11.6, 7.6 and 9.7%, respectively, sensitivity 15 pg/mL.
Statistical analysis
Statistical power calculations were computed using STATA, Version 8 (College Station, TX) with “sampsi” and “sampclus” commands to evaluate the pattern of postmenopausal depression for the four subgroups defined above. Each woman contributed numerous visits prior to menopause and a mean number of four visits postmenopause. Based on assumptions of 2-sided tests with type I alpha error of 5%, a mean of 8 repeated measures per participant, and a correlation of 0.4 among the repeated measures, the study has 80% power to detect a 50% decline (odds ratio = 0.50) in the prevalence of depressive symptoms postmenopause for women with a history of depression and a 66% decline (odds ratio = 0.33) for women whose depressive symptoms first occurred in the study prior to the FMP. There is 80% power to detect an odds ratio >= 0.33 in postmenopausal rates of depressive symptoms compared between women whose depressive symptoms first occurred in the study and women with no symptoms. Because the number of women who reported a history of depression was greater than the number of women who first experienced depressive symptoms in the study, we can detect a smaller odds ratio when comparing postmenopausal depressive symptoms between these two groups.
High CES-D scores ( >=16) were identified in each study year. All available data were included in analysis. The 203 participants had 2,514 CES-D observations. We present three distinct models in Tables 2–4 to address specific hypotheses regarding the prevalence of high CES-D scores (CES-D>=16 versus <16) as a function of time. All are versions of generalized linear mixed effects regression models. Details of model assumptions and methods are in supplemental material, Appendix A. For all models, observations during pregnancy, breast feeding and hormone use were censored. Covariates were defined a priori and added to the basic model singly and with an interaction with time. All covariates and interactions with time were further evaluated in multivariable models to determine their independent contributions.
Table 2.
Risk of depressive symptoms per year before and after the FMP
| History of depression, n=90 | No history of depression, n=113 | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Odds Ratio | 95% CI | P Value | N | Odds Ratio | 95% CI | P Value | |
| Years | 0.0041 | 0.0111 | ||||||
| <=−10 | 53 | 2.54 | 1.16 – 5.56 | 0.02 | 92 | 1.11 | 0.45 – 2.77 | 0.82 |
| −9 | 45 | 1.92 | 0.94 – 3.93 | 0.07 | 56 | 1.82 | 0.73 – 4.50 | 0.20 |
| −8 | 58 | 1.68 | 0.91 – 3.09 | 0.10 | 85 | 1.96 | 0.87 – 4.44 | 0.11 |
| −7 | 67 | 1.43 | 0.74 – 2.78 | 0.29 | 104 | 1.81 | 0.84 – 3.92 | 0.13 |
| −6 | 76 | 1.22 | 0.67 – 2.22 | 0.51 | 102 | 1.12 | 0.55 – 2.30 | 0.76 |
| −5 | 79 | 1.51 | 0.86 – 2.66 | 0.15 | 95 | 1.46 | 0.64 – 3.34 | 0.32 |
| −4 | 80 | 1.28 | 0.72 – 2.28 | 0.40 | 102 | 1.19 | 0.58 – 2.46 | 0.63 |
| −3 | 87 | 0.88 | 0.51 – 1.51 | 0.64 | 101 | 1.58 | 0.78 – 3.20 | 0.21 |
| −2 | 84 | 1.54 | 0.88 – 2.69 | 0.13 | 105 | 1.04 | 0.52 – 2.09 | 0.92 |
| −1 | 80 | 1.06 | 0.63 – 1.77 | 0.84 | 97 | 0.67 | 0.35 – 1.29 | 0.23 |
| FMP | Reference | ----- | ----- | ----- | ----- | ----- | ||
| 1 | 82 | 0.59 | 0.35 – 0.99 | 0.05 | 99 | 0.91 | 0.46 – 1.79 | 0.78 |
| 2 | 67 | 0.59 | 0.35 – 0.99 | 0.05 | 73 | 0.79 | 0.34 – 1.83 | 0.58 |
| 3 | 57 | 0.44 | 0.24 – 0.83 | 0.01 | 62 | 0.26 | 0.08 – 0.88 | 0.03 |
| 4 | 39 | 0.45 | 0.23 – 0.90 | 0.02 | 46 | 0.11 | 0.01 – 0.88 | 0.04 |
| 5 | 26 | 0.34 | 0.12 – 0.87 | 0.03 | 23 | 0.332 | 0.11 – 0.98 | 0.05 |
| 6 | 26 | 0.39 | 0.17 – 0.91 | 0.03 | 26 | ----- | ----- | ----- |
| 7 | 18 | 0.24 | 0.08 – 0.71 | 0.01 | 15 | ----- | ----- | ----- |
| ≥8 | 23 | 0.50 | 0.24 – 1.05 | 0.07 | 17 | ----- | ----- | ----- |
Wald statistic for overall test of significance
Subjects with no history of depression are combined in Years 5–8 due to low numbers for CES-D ≥ 16 (year 5, N=0; year 6, N=4; year 7, N=1; years 8 – 11, N=0).
Table 4.
Risk of high depressive symptoms postmenopause by occurrence before the FMP
| Odds Ratio | 95% CI | PValue | |
|---|---|---|---|
| Depression status in study | <0.0011 | ||
| Previous history of depression | 8.39 | 3.66 – 19.24 | <0.001 |
| 1st depression in study | |||
| Premenopause | 1.30 | 0.39 – 4.33 | 0.67 |
| Menopause transition | 2.39 | 0.87 – 6.59 | 0.09 |
| No depression before FMP | Reference | ----- | ----- |
| Time | |||
| >=2 years postmenopause | 0.51 | 0.37 – 0.71 | 0.001 |
| < 2 years postmenopause | Reference | ----- | ----- |
| Current medication | 1.89 | 1.15 – 3.12 | 0.03 |
Wald statistic for overall test of significance.
A second set of analyses focused on the rates of depressed mood postmenopause and evaluated associations with covariates and rates of change in covariates defined from the premenopausal time frame. Data from 179 participants and 685 post-menopausal CES-D observations were included in this set of analyses. The models included a random intercept only, with variance estimates for the statistical tests on the regression coefficients adjusted for repeated observations from each participant using generalized estimating equations.24
Hormone values were modeled using natural log transformations to reduce the influence of skewed distributions. The rate of change (slope) before the FMP was calculated for each hormone for each woman as the linear regression line for all points in the observed linear range prior to the FMP. The linear ranges were identified by inspection of the mean slope for the log hormone values for each year from baseline to the FMP and were 3 years for estradiol, 4 years for FSH, and 6 years for inhibin b. These were consistent with those previously identified by Zheng et al, who utilized a piecewise linear mixed effects model to identify timing changes in hormone trajectories.25 Hormone values are presented as geometric means with 95% confidence intervals. Odds ratios for hormones are presented per unit (1 standard deviation) increase in the rate of change (slope) for the log hormone.
All analyses were conducted using the SAS 9.3 statistical package (SAS, Inc, Cary, NC). Statistical tests were two-sided, with P<0.05 considered significant.
RESULTS
Sample description
Participants (N=203) were followed for 14 years and contributed a mean of 12.4 CES-D observations per woman. The mean age was 42.77 (SD 3.12) years at baseline and 51.10 (SD 3.30) years at the final menstrual period (FMP) (range 42–58 years); 47 % were African American and 53% white; 44% percent had a history of depression at cohort enrollment. Table 1 shows the baseline characteristics.
Pattern of CES-D scores around menopause
The overall prevalence of high CES-D scores decreased from 10 years before to 8 years following the FMP, with an overall decrease of approximately 15% per year (odds ratio 0.85, 95% CI: 0.81–0.89, P<0.001). When risk was compared to the FMP (Table 2), the risk of depressive symptoms was greater in each year before and lower in each year following the FMP. Repeating these models with a higher cut point of CES-D >=25 had similar results. The average decrease was approximately 12% per year (odds ratio 0.88, 95% CI: 0.83–0.93, P<0.001), and women with a history of depression were more than 11 times more likely to have high depressive symptoms (odds ratio =11.61, 95% CI: 5.51–24.47, P<0.001).
The pattern of depressive symptoms was strongly modified by a history of depression. Women with a history of depression were over 13 times more likely to have high depressive symptoms compared to women with no history of depression (odds ratio 13.62, 95% CI: 7.20–25.79, P<0.001). Table 2 shows the risk of depressive symptoms relative to the FMP by history of depression in each year. Both groups had a greater risk of depressive symptoms in the years before the FMP and less risk of depressive symptoms each year after the FMP. There was no significant interaction between a history of depression and CES-D over the study interval (P=0.08).
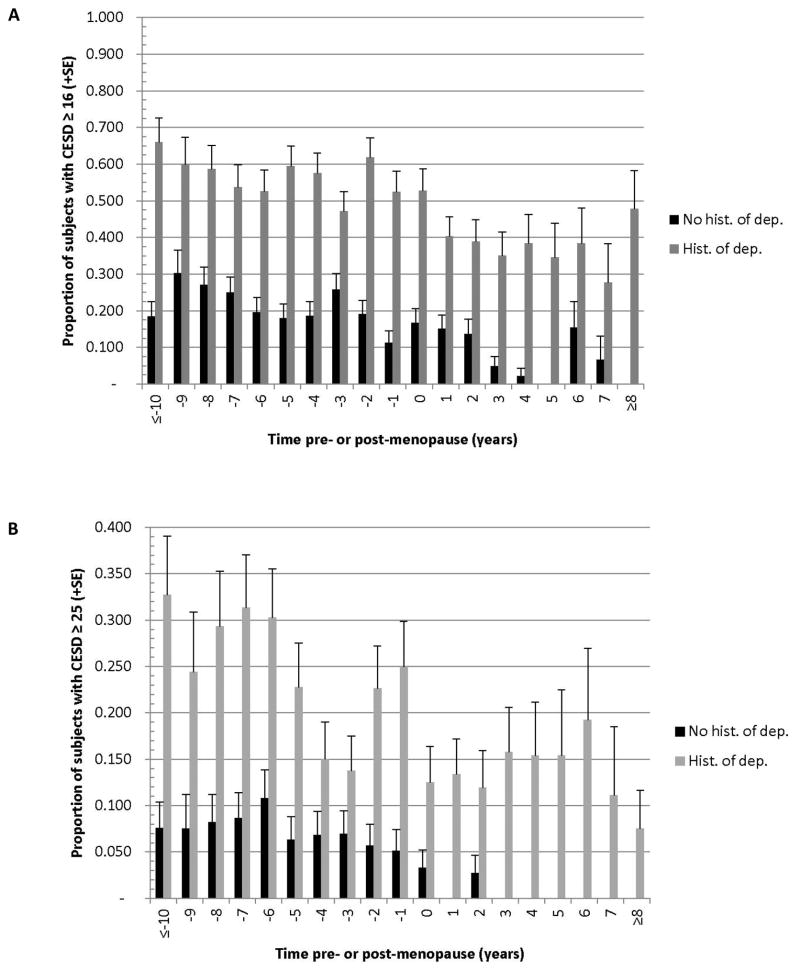
Figure 1A depicts the proportion of women with high CES-D in each study year by history of depression. Fifty to 65% of women with a history of depression had CES-D >=16 in each year before the FMP; this decreased to approximately 35% in each postmenopausal year. Among women with no history of depression, 10% – 30% had high CES-D before the FMP, with significant decrease after the second year postmenopause, when the incidence dropped to zero to 15%. No women in this group had CES-D >=25 postmenopause, except in year 2 (2 of 73 women, shown in Figure 1B).
Figure 1.
Proportion of women with CES-D ≥16 (A) or ≥25 (B) in each study year by history of depression at study enrollment. History of depression, N=90; no history of depression, N=113. Years at the range ends were combined due to small numbers of observations; the full range is 12 years before to 11 years after the FMP Results were generated from a multivariable generalized linear mixed model with robust variance (GEE model). Comparisons between the 2 groups each year are P<0.001.
Other risk factors
Table 3 shows associations of the a priori covariates with CES-D in time-adjusted analysis. Current smokers were approximately 2 times more likely to report high CES-D in the study interval compared to non-smokers (odds ratio 2.18, 95% CI: 0.99–4.79, P=0.05). Race, age at menopause and BMI were not significantly associated with CES-D scores. When these variables were adjusted for the presence of the other variables in multivariable analysis, only history of depression remained significantCurrent medication users were more likely to have high CES-D scores (odds ratio 2.87, 95% CI: 1.63–5.06, P=0.003). Inclusion of current medication in multivariable analysis did not change other estimates in the model, indicating that medication use was an independent risk factor marking high depressive symptoms but did not confound other associations in the study.
Table 3.
Time adjusted associations of each covariate with elevated CES-D scores,1 N=203
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Time 2 | 0.85 | 0.81 B 0.89 | <0.001 |
| History of depression | 13.62 | 7.20 B 25.80 | <0.001 |
| Current medication | 2.87 | 1.63 – 5.06 | 0.003 |
| Current smoking | 2.18 | 0.99 B 4.79 | 0.05 |
| Race | ----- | ----- | |
| African American | 1.68 | 0.81 B 3.48 | 0.17 |
| White | Ref. | ||
| Age at menopause >= 51.5 yrs | 0.61 | 0.29 B 1.26 | 0.18 |
| BMI ≥ 30 | 1.06 | 0.51 B 2.21 | 0.88 |
The association of each variable with CES-D ≥16, adjusted for time.
Unadjusted association per year of time with CES-D ≥16.
Prediction of depressive symptoms postmenopause
We further evaluated the postmenopausal risk of high depressive symptoms. Depressive symptoms were approximately 50% more likely in the first 2 years postmenopause than in subsequent years (odds ratio 0.51, 95% CI: 0.37–0.71, P=0.001) (Table 4). We then estimated the likelihood of depressive symptoms postmenopause by depression status in four categories as defined above. Women who had a history of depression were 8 times more likely to have depressive symptoms postmenopause than women who had no depression before the FMP (odds ratio 8.39, 95% CI: 3.66–19.24, P<0.001). In contrast, women whose first depressive symptoms occurred in the study had no significantly greater likelihood of depressive symptoms postmenopause than women who had no depression (odds ratio 1.30, 95% CI: 0.39–4.33, P=0.67 for women who were premenopausal and odds ratio 2.39, 95% CI: 0.87–6.59, P=0.09 for women in the menopause transition when depressive symptoms occurred) ( Table 4).
Hormone associations
We estimated the rate of change in FSH, estradiol, inhibin b before the FMP as predictors of depressive symptoms postmenopause. The risk of depressive symptoms after the FMP decreased by 35% for each unit (SD) increase in log FSH rate of change in multivariable analysis (odd ratio 0.65, 95% CI: 0.46–0.91, P=0.01) (Table 5, Model 2). A sensitivity analysis of the same model with removal of the most extreme 5% (N=7) log FSH slope observations had consistent but less significant results (odds ratio 0.75, 95% CI: 0.58–1.12). There was no significant association between the rate of change of estradiol or inhibin b and depressive symptoms following the FMP.
Table 5.
FHS rate of change before FMP as a predictor of postmenopausal depressive symptoms
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio1 | 95% CI | P Value | |
| FSH rate of change (4 yrs) | 0.631 | 0.46 – 0.84 | 0.004 | 0.651 | 0.46 – 0.91 | 0.01 |
| Time postmenopause | ||||||
| ≥ 2 yrs | <0.001 | 0.49 | 0.35 – 0.69 | <0.001 | ||
| < 2 yrs | Reference | ----- | ----- | ----- | ||
| Depression status | ----- | ----- | 0.0012 | |||
| History of depression | ----- | ----- | 6.52 | 2.76 – 15.42 | <0.001 | |
| 1st depression in study | ----- | ----- | ||||
| Premenopause | ----- | ----- | 1.12 | 0.34 – 3.65 | 0.85 | |
| Menopause transition | ----- | ----- | 1.61 | 0.56 – 4.61 | 0.38 | |
| No depression before FMP | ----- | ----- | Reference | |||
| Current Medication | ----- | ----- | 1.98 | 1.14 – 3.44 | 0.02 | |
Odds ratio indicates the likelihood of postmenopausal depressive symptoms for each 1 SD(.33) increase in the rate of change (slope) for log hormone FSH.
Wald statistic for overall test of significance.
DISCUSSION
This study identified a longitudinal pattern of depressive symptoms around natural menopause that showed symptoms decreased steadily from approximately 10 years before to 8 years following the FMP. The FMP was pivotal in this pattern, with greater risk of depressive symptoms before the FMP and lower risk of depressive symptoms after the FMP. The greater risk of depressive symptoms before the FMP is consistent with reports from our cohort and others that indicate an increased risk of depressive symptoms in the menopause transition.1–4, 6, Howeer, this study further shows that depressive symptoms decreased postmenopause compared to their levels at the FMP.
A history of depression strongly modified the incidence of depressive symptoms, with odds for high depressive symptoms nearly 13 times greater for women with a history of depression compared to women with no depression history. Approximately 50% to 65% of women with history of depression had high CES-D in each year before the FMP, and this decreased to approximately 35% with high CES-D in each postmenopausal year. In contrast, 10% to 30% of women with no history of depression had high CES-D in the years preceding the FMP, and the incidence decreased to zero to 15% after the second year postmenopause. This suggests that a risk of depressive symptoms for this group who first experienced the symptoms in the menopause transition continued for several years after the FMP but then sharply decreased, with a likelihood of depressive symptoms that was not significantly greater than the risk for women who had no depressive symptoms prior to menopause.
In our previous studies, we showed that greater variability (SD) in FSH and estradiol was significantly associated with depressive symptoms in the menopause transition.1, 3 Here we show that a faster rate of change in FSH before the FMP predicted a lower risk of depressive symptoms following the FMP, both in women with and without a history of depression. We speculate that women with a more rapid rise in FSH had a faster transition to menopause, followed by stable hormone levels that resulted in fewer depressive symptoms, but further studies are needed to more fully investigate this.
Several limitations are noted. Depressive episodes are often time limited and may not have been fully identified at annual evaluations. The CES-D with a cut point at >=16 is a standard measure of depressive symptoms and may not indicate a clinical diagnosis of depressive disorder, although it is noteworthy that results were similar with a higher cut point of CES-D >=25. We evaluated several covariates of depressive symptoms, but other factors likely exist. To extend interpretations of these findings, we are investigating longitudinal patterns of sleep disturbance and vasomotor symptoms in order to evaluate their joint relationships with depression. Finally, studies are needed to extend or refute these findings of a decreased risk of depression postmenopause, particularly for women who had no history of depression before the menopause transition.
Major strengths of the study include the analytic approach that evaluated within-woman changes in depressive symptoms relative to the FMP for each of 10 years before and 8 years after natural menopause in a population-based sample. All women had a premenopausal baseline and reached menopause during the study, thereby providing a clear identification of menopause with minimal recall bias. Longitudinal measures defined the rate of change (slope) in reproductive hormones before the FMP to uniquely evaluate the rate of change prior to menopause as predictors of depressive symptoms after the FMP.
Although only a minority of women experience mood difficulties in relation to menopause, many women want to know what to expect in this transition period. Women overall can expect depressive symptoms to decrease after the FMP, although those with a history of depression have a continuing high risk of recurrence However, women whose first experience of depressive symptoms occurred as they approached menopause can expect a low risk of depressive symptoms after the second year postmenopause. Clinician review of depressive symptoms is needed to provide treatment when symptoms are debilitating and to evaluate the impact of depression on other major disorders, such as cardiovascular disease, metabolic syndrome and osteoporosis. 26 Women with a history of depression may benefit from an anti-depressant or psychotherapy appropriate for a chronic disorder. However, women with no history of depression may have a low risk of depressive symptoms after the second postmenopausal year and benefit from short-term hormone therapy 27 or short-term treatments with anti-depressants that have demonstrated efficacy for menopausal symptoms28 Further studies to confirm these findings and elucidate the risk of depressive symptoms in later postmenopausal years are warranted.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health, grants RO1 AG12745 (to EWF, principal investigator) and RR024134 (to the Translational and Clinical Research Center, School of Medicine).
Footnotes
Dr. Freeman (principal investigator) and Dr. Sammel (biostatistician) had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest disclosures
Dr. Freeman reported research support (issued to the University of Pennsylvania) from Forest Laboratories, Inc. Bionovo, Inc, and Xanodyne Pharmaceuticals, Inc.
Dr. Sammel reported honoraria for lectures from the University of Rochester and Arcadia College; travel and meeting expenses from the University of Alabama Birmingham and The North American Menopause Society; consultant for Swiss Precision Diagnostics; statistical editor for the American Journal of Obstetrics and Gynecology.
Mr. Boorman reported no conflicts.
Dr. Zhang reported no conflicts. Dr. Zhang’s work on this project was during her doctoral training at the University of Pennsylvania.
Role of the sponsor: The NIH funded the grant application. It had no further role in the design and conduct of the study, including data collection, analysis and interpretation of the data, and the preparation, review and approval of the manuscript.
References
- 1.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 4.Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg EM, Rubinow DR, Bartko JJ, Fortinsky PM, Haq N, Thompson K, et al. A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry. 2008;69(6):973–980. doi: 10.4088/jcp.v69n0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161(12):2238–2244. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 7.Colangelo LA, Craft LL, Ouyang P, Liu K, Schreiner PJ, Michos ED, et al. Association of sex hormones and sex hormone-binding globulin with depressive symptoms in postmenopausal women: the Multiethnic Study of Atherosclerosis. Menopause. 2012;19(8):877–885. doi: 10.1097/gme.0b013e3182432de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez AD, Murray CC. The global burden of disease, 1990 – 2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 9.Schnatz PF, Nudy M, Shively CA, Powell A, O’Sullivan DM. A prospective analysis of the association between cardiovascular disease and depression in middle-aged women. Menopause. 2011;18(10):1096–1100. doi: 10.1097/gme.0b013e3182184928. [DOI] [PubMed] [Google Scholar]
- 10.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med. 2009;71(3):266–272. doi: 10.1097/PSY.0b013e318197a4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20(8):367–373. doi: 10.1016/j.tem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yirmiya R, Bab I. Major depression is a risk factor for low bone mineral density: a meta-analysis. Biol Psychiatry. 2009;66(5):423–432. doi: 10.1016/j.biopsych.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Psychol Med. 2011;41(9):1879–1888. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15(2):223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 15.Dennerstein L, Guthrie JR, Clark M, Lehert P, Henderson VW. A population-based study of depressed mood in middle-aged, Australian-born women. Menopause. 2004;11(5):563–568. doi: 10.1097/01.gme.0000113844.74462.f6. [DOI] [PubMed] [Google Scholar]
- 16.Gibson CJ, Joffe H, Bromberger JT, Thurston RC, Lewis TT, Khalil N, et al. Mood symptoms after natural menopause and hysterectomy with and without bilateral oophorectomy among women in midlife. Obstet Gynecol. 2012;119(5):935–941. doi: 10.1097/AOG.0b013e31824f9c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.QuickStats: Prevalence of Current Depression* Among Persons Aged 12 Years, by Age Group and Sex — United States, National Health and Nutrition Examination Survey, 2007 – 2010. MMWR Weekly. 2012;60(51):1747. [Google Scholar]
- 18.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98(3):391–397. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging. 1997;12(2):277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 21.Harlow BL, Cohen LS, Otto MW, Speigelman D, Cramer DW. Prevalence and predictors of depressive symptoms in older premenopausal women. Arch Gen Psychiatry. 1999;56:418–424. doi: 10.1001/archpsyc.56.5.418. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994 Dec;272(22):1749–1756. [PubMed] [Google Scholar]
- 23.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19(4):387–395. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang KY, Leger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 25.Zheng H, Sowers M, Randolph JF, Harlow SD. An integrated quantitative methodology to longitudinally characterize complex dynamic processes associated with ovarian aging and the menopausal transition. Journal on Systemic, Cybernetics and Informatics (JSCI) 2011;9 (3):15–23. [PMC free article] [PubMed] [Google Scholar]
- 26.Lutwak N, Dill C. The importance of screening and treating depression in all women. J Womens Health. 2012;21(12):1302. doi: 10.1089/jwh.2012.4042. [DOI] [PubMed] [Google Scholar]
- 27.Pinkerton JV, Stovall DW, Kightlinger RS. Advances in the treatment of menopausal symptoms. Womens Health. 2009;5(4):361–384. doi: 10.2217/whe.09.31. [DOI] [PubMed] [Google Scholar]
- 28.North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.