Abstract
Background. Specific antiretroviral therapy (ART) medications and the severity of human immunodeficiency virus (HIV) disease before treatment contribute to bone mineral density (BMD) loss after ART initiation.
Methods. We compared the percentage change in BMD over 96 weeks in 328 HIV-infected, treatment-naive individuals randomized equally to tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) plus atazanavir/ritonavir (ATV/r), darunavir/ritonavir (DRV/r), or raltegravir (RAL). We also determined whether baseline levels of inflammation markers and immune activation were independently associated with BMD loss.
Results. At week 96, the mean percentage changes from baseline in spine and hip BMDs were similar in the protease inhibitor (PI) arms (spine: −4.0% in the ATV/r group vs −3.6% in the DRV/r [P = .42]; hip: −3.9% in the ATV/r group vs −3.4% in the DRV/r group [P = .36]) but were greater in the combined PI arms than in the RAL arm (spine: −3.8% vs −1.8% [P < .001]; hip: −3.7% vs −2.4% [P = .005]). In multivariable analyses, higher baseline concentrations of high-sensitivity C-reactive protein, interleukin 6, and soluble CD14 were associated with greater total hip BMD loss, whereas markers of CD4+ T-cell senescence and exhaustion (CD4+CD28−CD57+PD1+) and CD4+ T-cell activation (CD4+CD38+HLA-DR+) were associated with lumbar spine BMD loss.
Conclusions. BMD losses 96 weeks after ART initiation were similar in magnitude among patients receiving PIs, ATV/r, or DRV/r but lowest among those receiving RAL. Inflammation and immune activation/senescence before ART initiation independently predicted subsequent BMD loss.
Keywords: bone mineral density, protease inhibitor, integrase inhibitor, human immunodeficiency virus, inflammation
Osteoporosis is common in human immunodeficiency virus (HIV)–infected populations, and emerging evidence suggests that fracture risk is higher in HIV-infected persons, compared with age-matched, HIV-uninfected controls [1, 2]. The first 48 weeks after antiretroviral therapy (ART) initiation has consistently been associated with a bone mineral density (BMD) loss of approximately 2%–6%, which does not return to baseline during continued treatment [3–6]. The magnitude of this BMD loss is dependent in part on the specific medications used. Among the nucleoside/nucleotide analogues, tenofovir disoproxil fumarate (TDF) has been consistently associated with an approximately 1%–2% greater loss in BMD during the period shortly after ART initiation [3–5]. The independent BMD effects of the third drug are less certain. Protease inhibitors (PIs) have been implicated in BMD loss after ART initiation, but most studies have examined PIs as a class [6, 7], rather than as individual medications, or have examined total body BMD [8, 9], rather than the more clinically relevant hip or spine BMDs. To our knowledge, only 1 study has compared the BMD effects of PIs to those of integrase inhibitors after ART initiation, and it found that elvitegravir/cobicistat was associated with slightly smaller decreases in BMD over 96 weeks, compared with atazanavir/ritonavir (ATV/r) [10].
Increased pretreatment HIV disease severity, defined on the basis of a lower baseline CD4+ T-cell count, is also associated with greater loss of BMD after ART initiation [9], and higher levels of the soluble tumor necrosis factor α (TNF-α) receptor 1 at baseline have been associated greater increases in the bone turnover markers c-telopeptide and osteocalcin, suggesting that increased inflammation at baseline may contribute to ART-associated bone loss [11]. Proinflammatory cytokines such as interleukin 6 (IL-6) and TNF-α are potent stimulators of osteoclast activity [12, 13], and in the general postmenopausal population, serum concentrations of IL-6 are major predictors of bone loss [14, 15]. The source of these cytokines may be activated or senescent T cells, which can be identified with cellular markers [16–18].
Activation of monocytes among HIV-infected persons has also been proposed as a major contributor to the pathogenesis of non-AIDS comorbidities [19, 20]. However, there are limited data investigating the relationship between monocyte activation and osteoporosis in HIV-infected persons [21], and the relationship between monocyte activation before ART initiation and BMD loss after ART initiation has not been reported.
Activated T cells and monocytes may influence bone resorption through alterations in the osteoprotegerin/receptor activator of nuclear factor-κ B ligand (RANKL) system [22], although activated T cells can also induce osteoclastic activity through osteoprotegerin/RANKL-independent pathways [23]. Osteoprotegerin and RANKL are osteoblast/osteocyte-secreted factors that have a major role in the coupling of bone formation and resorption, but they are also secreted by activated immune cells [12, 24, 25]. While these proteins act locally in the bone microenvironment, their circulating concentrations have been associated with osteoporosis in the general population [25]. We have found that higher osteoprotegerin concentrations had a protective effect on BMD in ART-naive HIV-infected persons [26], but whether these pre-ART levels of osteoprotegerin and RANKL influence bone loss after ART initiation is not clear.
The main objective of the current study was to determine whether BMD changes over 96 weeks after ART initiation differ in HIV-infected persons starting ATV/r, darunavir/ritonavir (DRV/r), or raltegravir (RAL) when combined with TDF/FTC. We also investigated whether BMD changes after ART initiation are related to baseline HIV-related variables and biomarkers related to inflammation, immune activation/senescence, monocyte activation, and bone regulation (osteoprotegerin/RANKL).
METHODS
A5260s was a substudy of AIDS Clinical Trials Group (ACTG) A5257, in which HIV-infected, ART-naive persons at least 18 years of age with an HIV type 1 (HIV) RNA load of ≥1000 copies/mL were randomized in an open-labeled fashion to receive TDF/FTC (300 mg/200 mg daily) plus either ATV/r (300 mg/100 mg daily), DRV/r (800 mg/100 mg daily), or RAL (400 mg twice daily). The primary end point of A5260s was subclinical cardiovascular disease (CVD). Therefore, subjects with known CVD or diabetes mellitus, uncontrolled thyroid disease, or use of lipid-lowering medications were excluded from participating. Randomization in A5257 was stratified by HIV RNA level, A5260s participation, and 10-year risk of myocardial infarction or death due to CVD. The preliminary CVD-associated results of A5260s have been reported elsewhere [27]. A secondary objective of A5260s was to compare changes in lumbar spine and total hip BMDs in the 3 treatment arms over 96 weeks. The parent study and substudy (clinical registration NCT00811954 and NCT00851799) were approved by the institutional review boards of all participating institutions, and all subjects provided written informed consent.
At baseline, information regarding demographic characteristics, health-related behaviors, medical conditions, and prescribed medications was obtained. BMD was assessed by dual-energy x-ray absorptiometry (DXA) of the lumbar spine (L1–L4) and total hip, using Hologic or Lunar scanners. Sites were instructed to use the same scanner and the same hip (left) at both study time points on the same subject. Total BMD was also assessed using whole-body DXA. All scans were read centrally by readers blinded to treatment assignment and clinical characteristics, using a standardized protocol at the Body Composition Analysis Center, Tufts University (Boston, Massachusetts). z scores were calculated from the site-specific BMD measurements, using normative data matched for age, sex, and race and, given the young age of the population, were used to summarize the baseline BMD data in accordance with National Osteoporosis Foundation guidelines [28].
Laboratory Assessment
Fasting blood samples (duration of fast, ≥8 hours) were obtained by phlebotomists and sent to core laboratories for analysis. Levels of soluble biomarkers were measured in plasma specimens stored at −70°C at the University of Vermont Laboratory for Clinical Biochemistry Research laboratory (Burlington). Levels of high-sensitivity C-reactive protein (hsCRP) were measured by nephelometry (interassay coefficient of variation [CV] range 2.96%–6.24%), and levels of soluble interleukin 2 receptor (IL-2R; (interassay CV range, 4.14%–9.51%), soluble CD14 (sCD14; interassay CV range, 11.24%–14.07%), soluble CD163 (sCD163; interassay CV range, 7.52%–8.72%), IL-6 (interassay CV range, 6.59%–12.48%), osteoprotegerin (interassay CV range, 8.74%–14.68%), and RANKL (interassay CV range, 10.05%–12.45%) were measured by enzyme-linked immunosorbent assay.
Immunophenotyping was performed using multicolor flow cytometry on cryopreserved peripheral blood mononuclear cells collected according to a standard ACTG protocol. The fluorochrome-conjugated antibodies used were anti-CD3 PE-Cy7 (clone SK7), anti-CD4 V450 (clone RPA-T4), anti-CD8 APC (clone RPA-T8), anti-CD8 APC-Cy7 (clone SK1), anti-CD28 PE-Cy 5 (clone CD28.2), anti-CD57 PE (clone NK-1), anti-CD279 (PD-1) APC (clone MIH4), anti-HLA-DR FITC (clone L243), anti-CD38 PE (clone HB7), anti-CD14 APC (clone M5E2), and anti-CD16 PE-Cy7 (clone 3G8; all from BD Biosciences). Data were analyzed in FlowJo software, version 9.3.3 (Treestar, Ashland, Oregon). The following monocyte phenotypes were characterized and expressed as percentages: proinflammatory phenotype (CD14+/CD16+) and nonclassical phenotype (patrolling monocytes; CD14dim/CD16+) [29]. CD4+ and CD8+ T cells with activated (HLA-DR+CD38+), senescent (CD28−CD57+), or senescent and exhausted (CD28−CD57+PD1+) phenotypes were assessed [30].
Statistical Analysis
The primary objective of this analysis was to determine whether the changes in lumbar spine or total hip BMD in the first 96 weeks after ART initiation differed between the randomized treatment arms. As a post hoc analysis, the 96-week percentage change in total BMD was also included as an outcome measure. Within-treatment-group changes in BMD were assessed with Wilcoxon signed rank tests. Per the study design, between-treatment-group comparisons used multivariable linear regression models with reverse Helmert contrasts, as follows. First, the ATV/r arm was compared to the DRV/r arm. If the difference was not statistically significant, the pooled PI/r arm was compared to the RAL arm. If the difference between the ATV/r and DRV/r arms was statistically significant, all pairwise comparisons between the treatment groups were performed. To account for multiple comparisons, all treatment comparisons were assessed with a type I error rate of 2.5%. All other statistical inferences were assessed with a 5% type I error rate. Analyses were adjusted for stratification factors (Framingham risk score and baseline HIV RNA level). Secondary during-treatment analyses that included only subjects who received their randomized treatment continuously for at least 96 weeks were also performed. Since the intention-to-treat (ITT) and during-treatment results were similar, only the ITT analyses are presented.
To evaluate whether baseline levels of soluble and cellular biomarkers were associated with the 96-week change in BMD, separate multiple linear regression models were used for each biomarker. Soluble biomarkers were log10 transformed prior to analysis. To standardize presentation, estimates of soluble biomarker levels are given per 0.3 log10 unit difference (equivalent to BMD effect per doubling of the biomarker level), and estimates of the effect of baseline cellular biomarker levels are presented as the change in BMD over 96 weeks for a 1% change in the biomarker, unless otherwise noted. All models were adjusted for age, race/ethnicity, sex, baseline body mass index (BMI), baseline CD4+ T-cell count, and baseline HIV RNA. All statistical analyses were performed with SAS (version 9.2, Cary, North Carolina).
RESULTS
Baseline Characteristics and Subject Disposition
A total of 334 subjects were enrolled from 26 US ACTG sites between June 2009 and April 2011. Of these subjects, 3 were subsequently found not to have met eligibility criteria (1 was not ART naive, 1 had virus with exclusionary nucleoside reverse transcriptase inhibitor [NRTI] mutations, and 1 was receiving a statin) and 3 discontinued the substudy follow-up within 1 day of enrollment, leaving 328 in the study analysis population (Table 1). The median BMD z score was −0.4 (interquartile range [IQR], −1.2 to 0.4) at the lumbar spine and −0.1 (IQR, −0.6 to 0.6) at the total hip. The percentage of subjects with low BMD (defined as a z score of −2.0 or less) was 9% at the lumbar spine and 1% at the total hip. At baseline, 21% reported receiving concomitant medications that affect bone, including androgens (n = 4), anticonvulsants (n = 1), proton-pump inhibitors (n = 22), corticosteroids (n = 8), estrogens (n = 5), tricyclic antidepressants (n = 3), and selective serotonin-reuptake inhibitors (n = 25). None of the subjects had a history of osteoporosis treatment. Concentrations of the biomarkers at baseline are presented in Table 1.
Table 1.
Subject Characteristics at Baseline
| Characteristic | ATV/r (n = 109) | RAL (n = 106) | DRV/r (n = 113) |
|---|---|---|---|
| Age, y | 37 (31–45) | 36 (27–44) | 35 (27–46) |
| Sex | |||
| Male | 91 | 89 | 89 |
| Female | 9 | 11 | 11 |
| Race/ethnicity | |||
| White | 49 | 41 | 42 |
| Black | 31 | 32 | 33 |
| Hispanic | 18 | 19 | 22 |
| Other | 2 | 8 | 3 |
| Weight, kg | 80 (69–88) | 77 (66–89) | 77 (67–83) |
| BMIa | 26 (23–29) | 24 (22–28) | 24 (22–27) |
| Concomitant medications affecting boneb | 22 | 22 | 20 |
| Current smoker | 40 | 37 | 36 |
| CD4+ T-cell count, cells/mm³ | 350 (211–461) | 343 (207–461) | 355 (207–461) |
| HIV RNA load, log10 copies/mL | 4.62 (4.05–5.10) | 4.52 (4.13–5.08) | 4.52 (3.95–4.95) |
| hsCRP level, mg/L | 1.45 (0.71–3.16) | 1.35 (0.69–2.8) | 1.17 (0.66–2.95) |
| IL-6 level, pg/mL | 1.82 (1.20–2.69) | 1.55 (1.07–3.02) | 1.78 (1.20–2.75) |
| sIL-2R level, pg/mL | 1862 (1479–2291) | 1820 (1202–2344) | 1660 (1202–2239) |
| sCD14 level, ng/mL | 1778 (1413–2138) | 1698 (1445–1950) | 1660 (1445–2042) |
| sCD163 level, ng/mL | 1148 (813–1585) | 1230 (832–1585) | 1023 (741–1548) |
| Osteoprotegerin level, pmol/L | 3.89 (3.16–4.90) | 3.98 (3.31–4.68) | 4.47 (3.63–5.25) |
| RANKL level, pg/mL | 35.5 (16.9–58.9) | 26.9 (11.2–53.7) | 24.5 (11.2–49.0) |
| Immunophenotype | n = 101 | n = 95 | n = 101 |
| CD28−CD57+, % of CD4+ T cells | 4.8 (2.2–9.9) | 5.2 (2.0–11.3) | 5.3 (2.6–9.3) |
| CD28−CD57+PD1+, % of CD4+ T cells | 0.03 (0.01–0.07) | 0.03 (0.01–0.07) | 0.03 (0.01–0.06) |
| CD28−CD57+, % of CD8+ T cells | 23.0 (16.9–30.5) | 25.9 (18.6–30.9) | 22.9 (18.4–30.5) |
| CD28−CD57+PD1+, % of CD8+ T cells | 0.07 (0.05–0.12) | 0.08 (0.05–0.16) | 0.09 (0.04–0.14) |
| CD38+HLA-DR+, % of CD4+ T cells | 19.0 (11.9–30.3) | 19.4 (12.0–32.2) | 17.4 (10.3–26.3) |
| CD38+HLA-DR+, % of CD8+ T cells | 41.7 (34.8–55.2) | 44.7 (37.0–53.7) | 42.5 (33.2–53.2) |
| CD14+CD16+, % of monocytes | 9.2 (5.8–15.1) | 7.8 (5.3–13.0) | 7.9 (5.7–12.1) |
| CD14lowCD16high, % of monocytes | 58.1 (47.3–69.4) | 62.5 (49.0–72.9) | 63.1 (53.6–72.2) |
Data are median values (interquartile ranges) or percentage of patients.
Abbreviations: ATV/r, atazanavir/ritonavir; DRV/r, darunavir/ritonavir; HIV, human immunodeficiency virus; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; RAL, raltegravir; RANKL, receptor activator of nuclear factor-κ B ligand; sCD14, soluble CD14; sCD163, soluble CD163; sIL-2R, soluble interleukin 2 receptor.
a Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
b Includes androgen, anticonvulsants, proton-pump inhibitors, corticosteroids, estrogens, and selective serotonin-reuptake inhibitors.
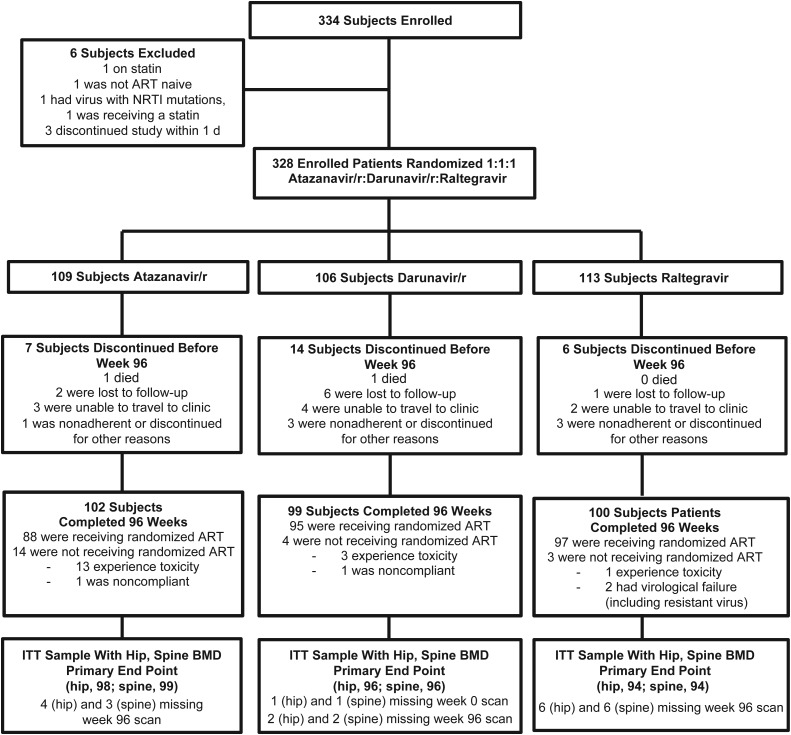
Over the 96-week follow-up period, 25 (8%) prematurely discontinued the substudy, and 2 died. Of the 301 who completed 96 weeks of study follow-up, 289 (96%) had 96-week DXA data at least 1 site, and 280 (93%) were receiving their randomized ART regimen at 96 weeks (Figure 1).
Figure 1.
Subject disposition. Abbreviations: ART, antiretroviral therapy; BMD, bone mineral density; ITT, intention to treat; NRTI, nucleoside reverse transcriptase inhibitor; r, ritonavir.
Changes in Bone Mineral Density Over 96 Weeks
Lumbar Spine
Lumbar spine BMD decreased significantly in each of the 3 treatment arms (all P < .001). The median percentage changes in lumbar spine BMD were as follows: ATV/r, −4.0% (IQR, −6.5 to −0.3); DRV/r, −3.1% (IQR, −5.2 to −0.8); and RAL, −1.6% (IQR, −3.6 to 0.9). The PI arms showed similar mean lumbar spine percentage BMD changes (−4.0% for ATV/r vs −3.6% for DRV/r; P = .42), but the pooled PI arms showed greater mean BMD loss than the RAL arm (−3.8% vs −1.8%; P < .001; Supplementary Figures 1A and 1B).
Total Hip
Total hip BMD decreased significantly in each of the 3 treatment arms (all P < .001). The median percentage changes in total hip BMD were as follows: ATV/r, −3.7% (IQR, −5.7 to −1.4); DRV/r, −3.3% (IQR, −5.2 to −0.9); and RAL, −2.2% (IQR, −4.5 to 0.4). Similar to the findings at the lumbar spine, no difference in the mean percentage BMD change at the hip from baseline to week 96 was apparent between the PI arms (−3.9% for ATV/r vs −3.4% for DRV/r; P = .36; Supplementary Figure 2A); the mean percentage BMD lost in the combined PI arms was greater than that in the RAL arm (−3.7% vs −2.4%; P = .005; Supplementary Figure 2B).
Total Body
The pattern of BMD changes in the total body BMD differed from the site-specific findings. While total body BMD decreased significantly in all 3 arms (P < .001), BMD loss was greater with ATV/r than DRV/r (−2.9% vs −1.6%; P = .001) and greater with ATV/r than RAL (−2.9% vs −1.7%; P = .004). No difference between the RAL and DRV/r arms was apparent (P = .72).
Effect of Baseline CD4+ T-Cell Count and HIV RNA Load
After adjustment for age, sex, race/ethnicity, baseline HIV RNA load, and BMI, no associations were detected between lower baseline CD4+ T-cell count and bone loss at the lumbar spine or total hip. In contrast, higher baseline HIV RNA load was associated with bone loss at both sites after multivariable adjustment (spine, −1.53% [95% confidence interval {CI}, −2.28% to −.77%] for each log10 copies/mL increase [P < .001]; total hip, −0.82% [95% CI, −1.51% to −.14%] for each log10 copies/mL increase [P = .02]).
Effect of Soluble Markers
Among the soluble markers (Table 2), higher hsCRP, IL-6, and sCD14 concentrations at baseline were associated with greater bone loss at the total hip at 96 weeks, after adjustment for age, sex, race, and baseline BMI, CD4+ T-cell count, and HIV RNA level. Higher sIL-2R concentrations showed a similar, albeit nonsignificant trend (P = .07). None of these markers were associated with bone loss at the lumbar spine. Associations of baseline sCD163, osteoprotegerin, or RANKL levels with bone loss at either the lumbar spine or the total hip were not detected (data not shown).
Table 2.
Associations Between Baseline Levels of Soluble and Cellular Markers and Bone Loss at 96 Weeks
| Marker | Spine BMD |
Hip BMD |
||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| hsCRPa | −0.02 (−.31 to .27) | .88 | −0.45 (−.70 to −.20) | <.001 |
| IL-6a | −0.16 (−.65 to .33) | .53 | −0.67 (−1.11 to −.24) | .002 |
| sIL-2Ra | −0.05 (−.86 to .75) | .89 | −0.66 (−1.38 to .07) | .07 |
| sCD14a | −0.57 (−2.18 to 1.04) | .49 | −1.67 (−3.10 to −.24) | .02 |
| sCD163a | 0.18 (−.54 to .90) | .62 | −0.04 (−.69 to .60) | .90 |
| Osteoprotegerina | −0.03 (−1.01 to .96) | .96 | −0.25 (−1.14 to .63) | .57 |
| RANKLa | 0.15 (−.12 to .41) | .28 | 0.20 (−.03 to .44) | .09 |
| CD4+CD28−CD57+b | −0.02 (−.32 to .28) | .91 | 0.09 (−.18 to .36) | .51 |
| CD4+CD28−CD57+PD1+a | −4.94 (−8.65 to −1.23) | .009 | −1.86 (−5.18 to 1.46) | .27 |
| CD8+CD28−CD57+b | 0.05 (−.19 to .29) | .68 | 0.06 (−.15 to .27) | .58 |
| CD8+CD28−CD57+PD1+a | 0.04 (−4.17 to 4.24) | .99 | 0.34 (−3.39 to 4.07) | .86 |
| CD4+CD38+HLA-DR+b | −0.26 (−.45 to −.06) | .01 | 0.02 (−.16 to .20) | .81 |
| CD8+CD38+HLA-DR+b | −0.01 (−.20 to .18) | .92 | 0.00 (−.17 to .18) | .98 |
All models are adjusted for age, sex, race, body mass index, baseline CD4+ T-cell count, and human immunodeficiency virus type 1 RNA load.
Abbreviations: BMD, bone mineral density; CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; RANKL, receptor activator of nuclear factor-κ B ligand; sCD14, soluble CD14; sCD163, soluble CD163; sIL-2R, soluble interleukin 2 receptor.
a Estimates represent the percentage change per doubling of the soluble biomarker level.
b Estimates represent the percentage change per 5% change in the cellular marker level.
c Estimates represent the percentage change per 1% change in the cellular marker level.
Effects of Cellular Markers
Among the cellular markers, a marker of CD4+ T-cell senescence and exhaustion (CD4+CD28−CD57+PD1+) and CD4+ T-cell activation (CD4+CD38+HLA-DR+) were associated with 96-week bone loss at the lumbar spine but not at the total hip in multivariable models (Table 2). Associations of cellular markers of T-cell senescence or activation on CD8+ T cells with bone loss at either site were not apparent. Similarly, associations between more-general populations of senescent T cells (CD4+CD28−CD57+ or CD4+CD28−CD57+) and bone loss at either site were also not observed. Associations of monocyte subpopulations (percentage of CD14+CD16+ monocytes, percentage of CD14lowCD16high monocytes) with bone loss at either the lumbar spine or the total hip or were also not detected (data not shown).
DISCUSSION
In this ART-naive, HIV-infected cohort initiating ART with TDF/FTC, we found that 96-week BMD losses at the lumbar spine and total hip were not different in the ATV/r and DRV/r arms. However, at these sites, BMD losses were less pronounced with RAL, compared with the PIs, suggesting that RAL may have a more neutral effect than PIs on bone. We also found that baseline markers of inflammation (hsCRP and IL-6 levels), monocyte activation (sCD14 level), and cellular markers of CD4+ T-cell immune activation (CD38+HLA-DR+) and senescence and exhaustion (CD28−CD57+PD1+) were associated with increased BMD loss, independent of baseline CD4+ T-cell count and HIV RNA level. Taken together, these findings indicate that the specific ART medications used and the degrees of baseline inflammation and immune activation are important determinants of bone loss after ART initiation.
To our knowledge, this is the first study to directly compare the effect of 2 PIs on BMD. Our finding of no difference in the 96-week loss in BMD between the PI arms suggests that the bone effects of these 2 PIs are equivalent. Early studies have shown that certain PIs induce osteoclastogenesis in in vitro models [31], but the specific mechanisms of new PIs, such as atazanavir and darunavir, have not been determined. Since all study subjects in the 2 PI arms also received TDF and ritonavir coadministration increases tenofovir concentrations by approximately 30% [32], it is possible that some of the PI effect observed in our study was related to an enhanced TDF effect on bone, rather than a specific PI effect. In contrast to this possibility, data from ACTG A5224s showed a similar PI effect (ATV/r) with either TDF/FTC or abacavir/lamivudine [4]. While the effects of ATV/r and DRV/r were similar at the clinically relevant lumbar spine and total hip, we did observe that persons randomized to ATV/r had greater total body bone loss, compared with those receiving DRV/r. The reasons and clinical significance for these latter findings are unclear. It is unlikely that the findings were due to differential dropout in the ATV/r arm, since the ITT and during-treatment analyses showed similar results. It is possible that these PIs have differential effects depending on the type of bone studied. The total body is about 80% cortical bone and 20% trabecular bone, whereas the spine is 20% cortical bone and 80% trabecular bone and the total hip is about 50% cortical bone and 50% trabecular bone [33]. It is possible that DRV/r has less of an effect on cortical bone, compared with ATV/r. However, we could not resolve these differences since DXA, unlike quantitative CT, cannot distinguish between trabecular and cortical bone.
In our study, those randomized to the integrase inhibitor, RAL, had significantly less bone loss at the lumbar spine and total hip, compared with those randomized to PIs. These data support the growing evidence that integrase inhibitors have minimal effect on bone after ART initiation [34] and complement findings from the SPIRAL study, which showed an increase in total BMD in participants who switched from a PI to RAL [35]. These findings suggest that, compared with PIs, RAL may be a better option for either initial or continued ART for persons at high risk of fragility fracture. There are fewer BMD data with other integrase inhibitors, but recent ART initiation trials with elvitegravir [10] or dolutegravir [36] also suggest a more neutral effect on bone metabolism, similar to RAL. Clearly, more studies with these agents are warranted.
In addition to the specific effects of ART, baseline levels of inflammation and immune activation were independently associated with bone loss after ART initiation. To our knowledge, our study is the first to demonstrate that well-known markers of inflammation, such as hsCRP and IL-6 levels, were associated with increased bone loss after ART initiation. Interestingly, these markers were associated with bone loss at the total hip but not the lumbar spine. Since we only measured BMD at 96 weeks and BMD tends to change more rapidly at the spine with a metabolic perturbation, it is possible that we may have missed a significant effect occurring earlier.
We also examined the effects of pretreatment concentrations of soluble and cellular markers of T-cell activation and monocyte/macrophage activation. We found that CD4+CD38+HLA-DR+, a cellular marker of CD4+ T-cell activation, and CD4+CD28−CD57+PD1+, a marker of T-cell senescence and exhaustion, were associated with bone loss at 96 weeks at the lumbar spine. Interestingly, in contrast to the findings with CD4+ T-cell subsets, we failed to detect associations between markers of CD8+ T-cell activation and senescence/exhaustion and bone loss. It is possible that activated CD4+ T cells are the main T cells that produce RANKL [37] and may be more-important mediators of bone loss [38]. In addition, senescent and exhausted CD4+ T cells may have impaired immune responses, and this may lead to reduced production of osteoprotegerin, which may protect against bone loss [39]. Our results regarding markers of monocyte activation and proinflammatory monocytes were mixed. Whereas higher levels of sCD14, a soluble marker of monocyte activation, were associated with bone loss at 96 weeks, levels of sCD163 or the proportion of proinflammatory monocyte subpopulations were not. Taken together, these findings further support the hypothesis that a pretreatment inflammatory set point determines the occurrence and severity of non-AIDS complications, including bone loss, independently of CD4+ T-cell count and HIV RNA load [40]. Our results support the hypothesis that earlier ART initiation or the initiation of targeted therapies aimed to reduce immune activation prior to ART initiation may be useful to mitigate the negative effects on BMD after ART initiation.
Our study had several limitations. Our study comprised mostly men and excluded populations with certain comorbid conditions associated with systemic inflammation (eg, diabetes mellitus and CVD), which may limit the generalizability of our findings. Second, all study participants received TDF/FTC, which has known negative effects on bone. Whether the PI or RAL effects would be different in the setting of a different NRTI backbone or combined with medication in a different ART class is unclear. Next, we only measured levels of selected markers of inflammation and bone metabolism. Further studies should also measure levels of other cytokines that have been implicated in inflammation-related bone loss, such as tumor necrosis factor α and its receptors. Finally, our findings regarding baseline biomarkers and bone loss should be considered hypothesis generating and need to be confirmed, given that the number of statistical tests performed increased the possibility of type 1 error.
In conclusion, we found that the PIs ATV/r and DRV/r led to a similar degree of bone loss over 96 weeks in persons initiating ART with TDF/FTC, which was significantly greater than the bone loss observed with RAL. We also found that higher pretreatment levels of selected inflammation and immune activation markers were associated with a greater degree of bone loss. These observations may have important implications for clinical care, suggesting that avoidance of PIs in favor of RAL may be a good strategy to minimize bone loss in ART-naive patients who have a high baseline risk of fracture. These findings also provide rationale to examine the effects of integrase inhibitor substitution on bone in HIV-infected persons who are receiving PI-containing ART and have a high fracture risk and to examine strategies to reduce pretreatment inflammation/immune activation, including earlier ART initiation, to preserve skeletal health.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH (grants HL095132, HL095126, AI 068636, AI068634, AI69471, and AI56933), the NIAID (award U01AI068636), the National Institute of Mental Health, and the National Institute of Dental and Craniofacial Research. The protocol received support from the AIDS Clinical Trials Group (ACTG), the ACTG Statistical and Data Management Center (grant UM1AI68634), the ACTG specialty laboratories listed in the article, and individuals and grants from the following 26 clinical research sites (CRSs) of the AIDS Clinical Trials Unit (ACTU) clinical research sites: Cincinnati CRS (site 2401; Michelle Saemann, RN, and Jennifer Baer, RN; ACTU grant AI069439), Ohio State University CRS (site 2301; Susan Koletar, MD, and Mark Hite, RN; ACTU grant UM1AI069494), Vanderbilt Therapeutics CRS (site 3652; Vicki Bailey, RN, and Rebecca Basham, CCRP; ACTU grant 2UM1AI069439-08 and Clinical and Translational Science Award [CTSA] UL1 TR000445), Chapel Hill CRS (site 3201; David Currin, RN, and Miriam Chicurel-Bayard, RN; ACTU grant UM1 AI069423-08, CTSA 1UL1TR001111, and Centers for AIDS Research [CFAR] grant P30 AI50410), Washington University Therapeutics CRS (site 2101; Teresa Spitz and Judy Frain; ACTU grant UM1AI069439), University of Colorado Hospital CRS (site 6101; Beverly Putnam and Cathi Basler; ACTU grant 2UM1AI069432 and CTSA grant UL1 TR001082), University of Southern California CRS (site 1201; Michael P. Dube, MD, and Bartolo Santos, RN; ACTU grant AI069432), Harbor–University of California–Los Angeles (UCLA) CRS (site 603; Eric Daar and Sadia Shaik; ACTU grant AI069424 and CTSA grant UL1TR000124), Northwestern University CRS (site 2701; Babafemi Taiwo, MBBS, and Baiba Berzins, MPH; ACTU grant 5U01 AI069471), UCLA CARE Center CRS (site 601; Emery Chang, MD, and Maria Palmer; ACTU grant A1069424), University of Rochester ACTG CRS/AIDS CARE CRS/Trillium Health (sites 1101 and 1108; Mary Adams, RN, and Christine Hurley, RN; ACTU grant 2UM1 AI069511-08 and CTSA grant UL1 TR024160), Ponce de Leon Center CRS (site 5802; Carlos del Rio, MD, and Ericka Patrick, RN; ACTU grant 2UM1 AI069418-08, CFAR grant P30 AI050409, and CTSA grant UL1 TR000454), Rush University CRS (site 2702; Beverly Sha, MD, and Veronica Navarro, RN; ACTU grant AI-069471), Boston Medical Center ACTG CRS (site 104; Benjamin Linus, MD; ACTU grant UM1 AI069472), Beth Israel Deaconess Medical Center ACTG CRS (site 103; Mary Albrecht, MD; ACTU grant UM1 AI069472 and CFAR grant P30 AI060354), Houston AIDS Research Team CRS (site 31473; Roberto C. Arduino, MD, and Maria Laura Martinez; ACTU grants 2 UM1 AI069503-08 and 2 UM1 AI068636-08), Brigham and Women's Hospital Therapeutics CRS (site 107; Paul Sax, MD, and Cheryl Keenan, RN, BC; ACTU grant UM1AI069472, CFAR grant P30 AI060354, and CTSA grant UL1 TR000170), MetroHealth CRS (site 2503; Kim Whitely, RN, and Traci Davis, RN; ACTU grant AI 69501 and CTSA grant UL1TR000439), New York University HIV/AIDS CRS (site 401; Judith A. Aberg, MD, and Michelle S. Cespedes, MPH, MD; ACTU grant UM1 AI069532), University of Washington AIDS CRS (site 1401; Shelia Dunaway, MD, and Sheryl Storey, PA-C; ACTU grant UM AI069481), Johns Hopkins University CRS (site 201; Joel Gallant, MD, and Ilene Wiggins, RN; ACTU grant 2UM1 AI069465 and CTSA grant UL1TR001079), University of California–San Francisco (UCSF) HIV/AIDS CRS (site 801; Annie Luetkemeyer, MD, and Jay Dwyer, RN; ACTU grant UM1 AI069496 and UCSF-CTSA grant UL1 TR000004), Case Western Reserve University CRS (site 2501; Kristen Allen, RN, and Patricia Walton, RN; ACTU grant AI069501), Duke University Medical Center Adult CRS (site 1601; Mehri McKellar, MD, and Jacquelin Granholm, RN; ACTU grant 5UM1 AI069484-07), and University of Pittsburgh CRS (site 1001; Sharon Riddler, MD, and Lisa Klevens, BSN; ACTU grant UM1 AI069494).
Potential conflicts of interest. T. T. B. has served as a consultant for BMS, GlaxoSmithKline (GSK), Merck, Abbott, Gilead, and ViiV Healthcare and has received research funding from Merck and GSK. J. S. C. has served as a consultant for Gilead and has received research funding from Merck. M. P. D. has served as a consultant for Gilead and Astra Zeneca and has received research funding from Gilead, Serono, and ViiV. R. L. M. has served as a consultant for Gilead and serves on a data safety monitoring board for Gilead. J. H. S. serves on a data safety monitoring board for Lilly and has a research grant from Gilead. G. A. M. has served as a consultant and/or speaker for and/or has received research grants from BMS, Pfizer, Merck, and GSK. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006; 20:2165–74. [DOI] [PubMed] [Google Scholar]
- 2.Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS 2013; 27:1949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–72. [DOI] [PubMed] [Google Scholar]
- 4.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 6.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009; 27:817–24. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet E, Ruidavets JB, Genoux A, et al. Early loss of bone mineral density is correlated with a gain of fat mass in patients starting a protease inhibitor containing regimen: the prospective Lipotrip study. BMC Infect Dis 2013; 13:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. [DOI] [PubMed] [Google Scholar]
- 9.Grant PM, Kitch D, McComsey GA, et al. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis 2013; 57:1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockstroh JK, DeJesus E, Henry K, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013; 62:483–6. [DOI] [PubMed] [Google Scholar]
- 11.Brown TT, Ross AC, Storer N, Labbato D, McComsey GA. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antivir Ther 2011; 16:1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwan TS, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 2004; 15:49–60. [DOI] [PubMed] [Google Scholar]
- 13.Siggelkow H, Eidner T, Lehmann G, et al. Cytokines, osteoprotegerin, and RANKL in vitro and histomorphometric indices of bone turnover in patients with different bone diseases. J Bone Miner Res 2003; 18:529–38. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab 2008; 93:1952–8. [DOI] [PubMed] [Google Scholar]
- 15.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab 2001; 86:2032–42. [DOI] [PubMed] [Google Scholar]
- 16.Effros RB. Replicative senescence of CD8 T cells: effect on human ageing. Exp Gerontol 2004; 39:517–24. [DOI] [PubMed] [Google Scholar]
- 17.Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol 1998; 33:267–82. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol 2005; 40:81–7. [DOI] [PubMed] [Google Scholar]
- 19.Barbour JD, Jalbert EC, Chow DC, et al. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis 2014; 232:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlandson KM, O'riordan M, Labbato D, McComsey GA. Relationships between inflammation, immune activation, and bone health among HIV-infected adults on stable antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 65:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ofotokun I, McIntosh E, Weitzmann MN. HIV: inflammation and bone. Curr HIV/AIDS Rep 2012; 9:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weitzmann MN, Cenci S, Rifas L, Haug J, Dipersio J, Pacifici R. T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand-dependent and -independent mechanisms. J Bone Miner Res 2001; 16:328–37. [DOI] [PubMed] [Google Scholar]
- 24.Chakravarti A, Marceau AA, Flamand L, Poubelle PE. Normal human primary CD4+ T lymphocytes synthesize and release functional osteoprotegerin in vitro. Lab Invest 2008; 88:171–84. [DOI] [PubMed] [Google Scholar]
- 25.Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab 2007; 92:4514–21. [DOI] [PubMed] [Google Scholar]
- 26.Brown TT, Chen Y, Currier JS, et al. Body composition, soluble markers of inflammation, and bone mineral density in antiretroviral therapy-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr 2013; 63:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein JH, Hodis H, Brown TT, et al. Prospective randomized clinical trial on the effects of three modern antiretroviral therapies on carotid intima-media thickness in HIV-infected individuals (AIDS clinical trial group study A5260s). J Am Coll Cardiol 2014; 63:A1322. [Google Scholar]
- 28.Cosman F, de Beur SJ, Leboff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25:2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116:e74–80. [DOI] [PubMed] [Google Scholar]
- 30.Lee SA, Sinclair E, Hatano H, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One 2014; 9:e89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem 2002; 277:19247–50. [DOI] [PubMed] [Google Scholar]
- 32.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr 2006; 43:278–83. [DOI] [PubMed] [Google Scholar]
- 33.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 2008; 3(suppl 3):S131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses 2012; 29:256–65. [DOI] [PubMed] [Google Scholar]
- 35.Curran A, Martinez E, Saumoy M, et al. Body composition changes after switching from protease inhibitors to raltegravir: SPIRAL-LIP substudy. AIDS 2012; 26:475–81. [DOI] [PubMed] [Google Scholar]
- 36.Tebas P, Kumar P, Hicks C, et al. 48 week bone marker changes with dolutegravir (DTG; GSK1349572) plus abacavir/lamivudine (ABC/3TC) vs. tenofovir/emtricitabine/efavirenz (EFV/TDF/FTC): the SINGLE trial [abstract H-1461] Presented at: 53rd Interscience Conference of Antimicrobial Agents and Chemotherapy, Denver, Colorado, 10–13 September 2013. [Google Scholar]
- 37.Jones DH, Kong YY, Penninger JM. Role of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis 2002; 61(suppl 2):ii32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng YT, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest 2000; 106:R59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008; 473:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.