Fig. 1.
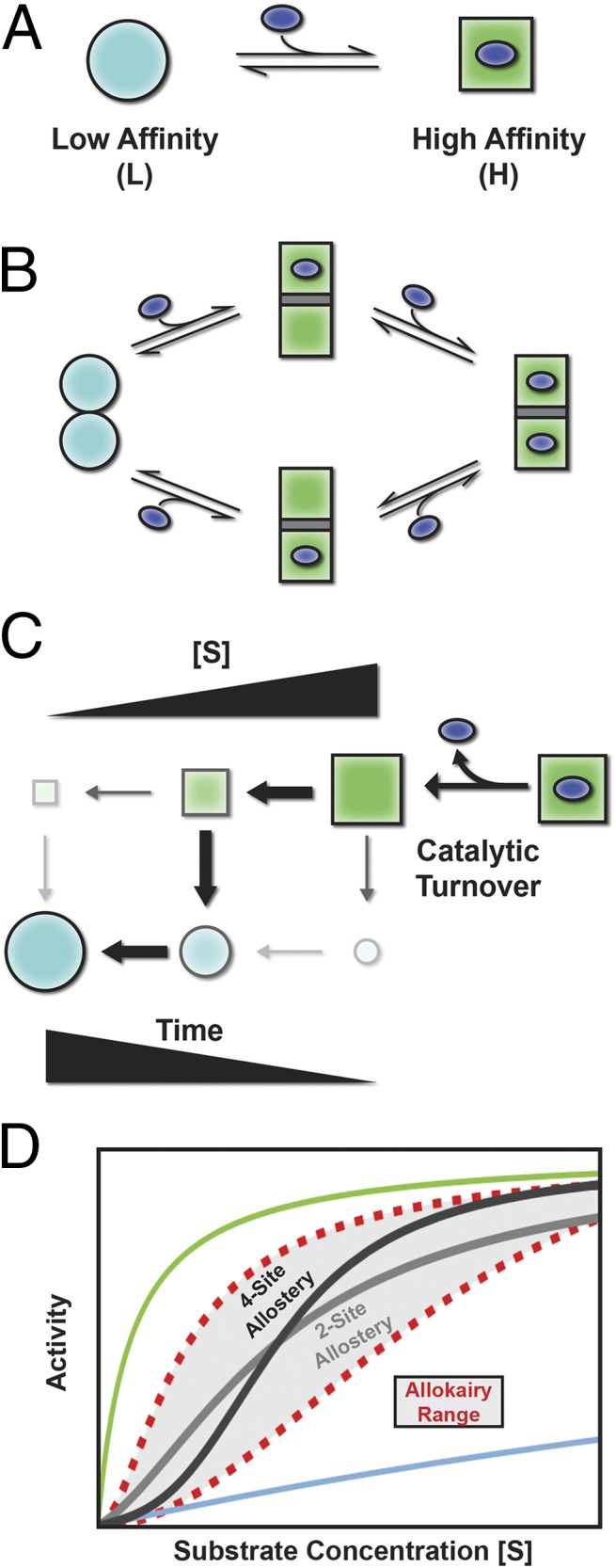
Allosteric and allokairic regulation. (A) Tunability requires that an enzyme can populate at least two states, depicted here as low-affinity (L) and high-affinity (H) with the ligand represented as the blue oval. (B) In allostery, binding and conformational change are coupled. Binding substrate to one molecule of a dimer, for example, converts both to the high-affinity H state, increasing the binding affinity. (C) In allokairy, the enzyme is in the H state after turnover (upper right) and relaxes back to the L state (lower left) determined by the time between turnover and binding, which depends on substrate concentration ([S]). (D) Allostery (two-site dimer, light gray; four-site tetramer, dark gray) and allokairy (red dashed lines) both lead to S-shaped curves when activity is plotted against substrate concentration. The curves for a fully L state or H state are shown in blue and green (top and bottom). Although both regulation mechanisms produce a sigmoidal change in activity, transitioning from the L state at low [S] to the H state at high [S], cooperativity in allokairy is more tunable (gray shading).
