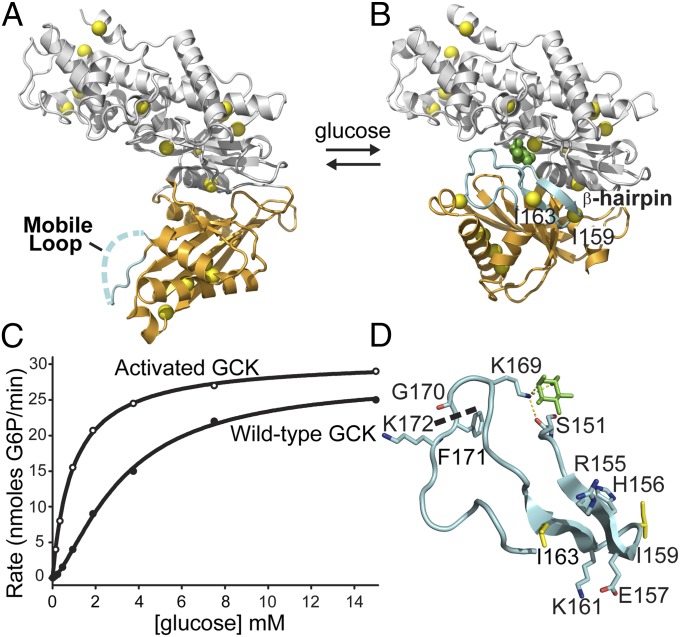
Fig. 1.
Conformational changes and kinetic profile of human GCK. (A) In the unliganded state, the mobile loop (cyan) displays no electron density and the small domain (orange) adopts a super-open conformation. (B) Upon glucose binding, the mobile loop folds into an antiparallel β-hairpin and K169 forms a hydrogen bonding network with glucose (green) and S151. Isoleucine residues (yellow) used as NMR reporters are evenly distributed throughout the molecule. (C) The sigmoidal kinetic response of GCK results from conformational rearrangements occurring on a timescale comparable to kcat. (D) View of the 151–180 loop, revealing the β-hairpin formed by residues 154–164 upon glucose binding (green). The thermolysin cleavage site is shown as a dashed line.