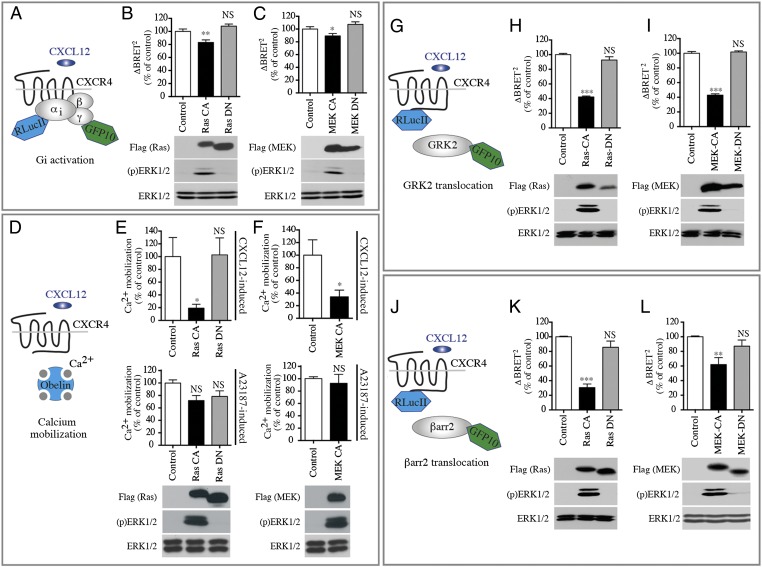
Fig. 1.
ERK/MAPK pathway activation reduces Gi activation and second-messenger production as well as GRK2 and βarr2 translocation in response to CXCR4 activation. (A) Schematic representation of the BRET-based ligand-induced Gi activation assay. (B and C) CXCL12-promoted Gi activation measured by BRET in HEK293T cells cotransfected with HA-CXCR4, Gαi1-RLucII, Gβ1, and Gγ2-GFP10, without (Control) or with either Flag-Ras CA or Flag-Ras DN (B) or Flag-MEK CA or Flag-MEK DN (C). BRET400-GFP10 between Gαi1-RLucII and Gγ2-GFP10 was measured after the addition of coel-400a, 3 min following the addition of CXCL12. Data are expressed as agonist-promoted BRET (ΔBRET; see Fig. S1A). (D) Schematic representation of the Obelin-based Ca2+ mobilization assay. (E and F) The increase in CXCL12-promoted intracellular calcium measured in HEK293T cells transfected with Obelin-Cherry in the absence (Control) or presence of the indicated Ras and MEK mutants. Luminescence was measured every second for 60 s after the injection of CXCL12 or calcium ionophore A23187. Bars represent the area under the curve calculated from the kinetics curves (Fig. S1B). (G and J) Schematic representations of the BRET-based ligand-induced GRK2 (G) and βarr2 (J) translocation. (H, I, K, and L) CXCL12-promoted BRET was measured in HEK293T cells transfected with CXCR4-RLucII and GRK2-GFP10 (H and I) or βarr2-GFP10 (K and L), in the absence (Control) or presence of the indicated Ras or MEK mutants. BRET400-GFP10 between CXCR4-RLucII and GRK2-GFP10 or βarr2-GFP10 was measured after the addition of coel-400a, 15 min after the addition of CXCL12. Data are expressed as agonist-promoted BRET (ΔBRET; see Fig. S1 C and D). In all cases, data shown represent the mean ± SEM of three independent experiments and were normalized to 100% of the control condition. Expression of CA or DN forms of Flag-Ras or Flag-MEK and the total ERK1/2 and ERK1/2 activation status [(p)ERK1/2], was assessed by immunoblotting. Representative experiments are shown below the graphs.*P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.