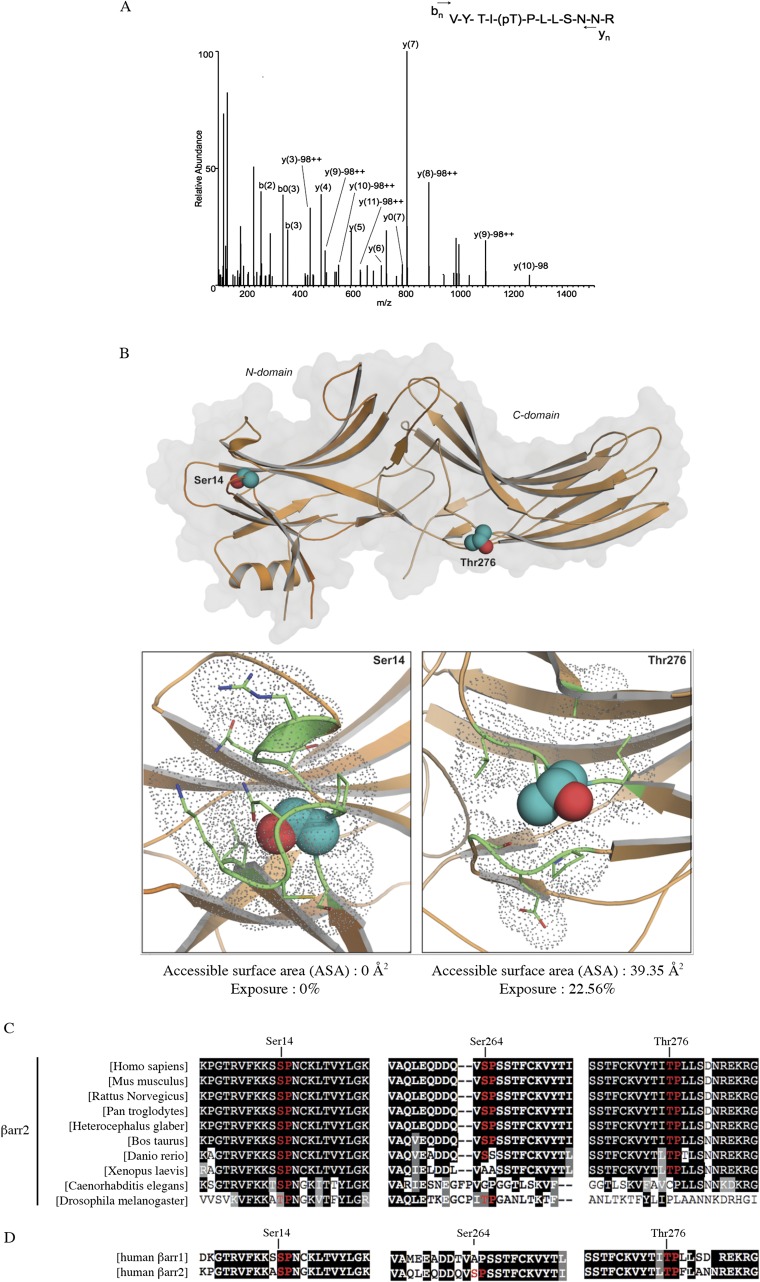
Fig. S3.
Ser14 and Thr276 residues of βarr2 are putative ERK1/2 phosphorylation sites. (A) LC-MS/MS of bovine βarr2: peptide VYTITPLLSNNR, MH+ 1469.721, m/z 735.8681, charge 2+. The characteristic peptide bond fragment ions, type b and y ions, are labeled. Eight microliters of sample were injected into a nanoflow Eksigent HPLC system for online C18 reversed-phase chromatographic separation coupled to an LTQ-Orbitrap Elite or Q Exactive mass spectrometer. The mass spectrometer was operated in the data-dependent mode, in which a full-scan MS was followed by MS/MS scans of the three most abundant ions with +2 to +4 charge states. (B) ERK1/2 putative phosphorylation sites Ser14 and Thr276. (Upper) Ribbon diagram of the βarr2 structure (PDB ID code 3P2D) depicted in orange. Putative sites for ERK1/2 phosphorylation (Ser14 and Thr276) are shown as spheres with carbon and oxygen atoms colored cyan and red, respectively. (Lower) Close-up of the structural environment of Ser14 and Thr276 putative phosphorylation sites. Side-chains of the amino acids in direct proximity of each site (within 4 Å) are highlighted in green and are shown as sticks and gray dots to represent their volume, illustrating the relative accessibility of each phosphorylation site. (C) Conservation between species of the three putative ERK1/2 phosphorylation sites in βarr2. (D) Conservation between human βarr1 and human βarr2 of the three putative ERK1/2 phosphorylation sites identified in βarr2.