Abstract
The rationale for our study was to determine the pattern of ethanol drinking by the high alcohol-drinking (HAD) replicate lines of rats during adolescence and adulthood in both male and female rats. Rats were given 30 days of 24 h free-choice access to ethanol (15%, v/v) and water, with ad lib access to food, starting at the beginning of adolescence (PND 30) or adulthood (PND 90). Water and alcohol drinking patterns were monitored 22 h/day with a “lickometer” set-up. The results indicated that adolescent HAD-1 and HAD-2 males consumed the greatest levels of ethanol and had the most well defined ethanol licking binges among the age and sex groups with increasing levels of ethanol consumption throughout adolescence. In addition, following the first week of adolescence, male and female HAD-1 and HAD-2 rats differed in both ethanol consumption levels and ethanol licking behavior. Adult HAD-1 male and female rats did not differ from one another and their ethanol intake or licking behaviors did not change significantly over weeks. Adult HAD-2 male rats maintained a relatively constant level of ethanol consumption across weeks, whereas adult HAD-2 female rats increased ethanol consumption levels over weeks, peaking during the third week when they consumed more than their adult male counterparts. The results indicate that the HAD rat lines could be used as an effective animal model to examine the development of ethanol consumption and binge drinking in adolescent male and female rats providing information on the long-range consequences of adolescent alcohol drinking.
Keywords: Adolescence, Adult, Alcohol-drinking patterns, High alcohol-drinking (HAD) rats, Selectively bred rats, Sex differences
1. Introduction
Age and sex differences in alcohol use and associated problems have been the focus of much prior research (For reviews, see Schulte et al., 2009; Masten et al., 2008; Vetter et al., 2007). Alcohol use typically begins in the second decade of life, with the first use of alcohol typically occurring in early adolescence (13–14 years of age: Faden, 2006). Results from the National Longitudinal Alcohol Epidemiological Survey revealed that individuals initiating alcohol use before the age of 14 had a four times higher rate of lifetime alcohol dependence than individuals that initiated use after the age of 20 (Grant and Dawson, 1997), implicating alcohol use during early adolescence in the development of later alcohol use disorders.
While drinking and alcohol use problems escalate throughout late adolescence (16–20 years of age: For review, see Brown et al., 2008), differences between the sexes generally do not occur until age 18 (Young et al., 2002). After this point, it has been shown that adult males consume more alcohol and have more alcohol related problems than females (Substance Abuse and Mental Health Services Administration, 2008), suggesting a developmental process during late adolescence which differentiates the drinking patterns and disorders seen between the sexes. Thus, research on this period of adolescence may reveal important information on why more adult men develop alcohol use disorders than women.
Experiments carried out in female alcohol preferring (P) rats revealed that exposure to alcohol during adolescence (Rodd-Henricks et al., 2002a), but not adulthood (Rodd-Henricks et al., 2002b), results in (a) quicker acquisition of operant self-administration of ethanol, (b) greater resistance to extinction of this behavior, and (c) greater relapse drinking when tested subsequently in adulthood. In another study, male adolescent BALB/cByJ mice given 2-bottle choice access to ethanol displayed increased ethanol preference in adulthood (Blizard et al., 2004). However, other studies have shown that forced administration via ethanol vapor or forced consumption during adolescence did not increase intake in adult Sprague–Dawley rats (Slawecki and Betancourt, 2002; Tolliver and Samson, 1991). A subsequent study found that Sprague Dawley rats that first voluntarily consumed alcohol during adolescence, when tested during adulthood at the same chronological age as a group of rats that first voluntarily consumed ethanol during adulthood, showed no difference between groups, indicating that in Sprague Dawley rats, alcohol exposure in adolescence, whether it be forced or voluntary, does not appear to affect ethanol consumption in adulthood (Vetter et al., 2007). Such studies indicate that certain rodent lines or strains provide better animal models for different developmental characteristics of alcoholism. In order to determine the best developmental model for ethanol consumption paralleling that seen in human males and females, the drinking patterns of different strains of both rodent sexes throughout these developmental periods must be measured. To date, there is only one published report of a detailed examination of drinking patterns that has taken into account age and sex of animal across weeks of free-choice ethanol access. That report was based on findings with the alcohol-preferring (P) rat (Bell et al., 2006).
For the P rat, Bell et al. (2006) found that rats increased alcohol consumption throughout adolescence. They also found that male rats in late adolescence consumed more alcohol than female rats in late adolescence, and ethanol-experienced male rats in adulthood consumed more alcohol than their female counterparts. Unlike this study (Bell et al., 2006), other studies have found that ethanol consumption decreased across the adolescent-to-adult transition (Vetter et al., 2007; Truxell et al., 2007; Doremus et al., 2005). In addition, it should be noted that the former study (Bell et al., 2006) is one of the few studies that has shown an adult rodent male to consume more ethanol than their adult female counterpart, with the majority of previous studies demonstrating the reverse (Adams, 1995; Juárez and Barrios de Tomasi, 1999; Lancaster and Spiegel, 1992; Li and Lumeng, 1984; Moore et al., 2010; Truxell et al., 2007; Vetter-O'Hagen et al., 2009). Although the patterns of ethanol consumption seen in the P rat differs from those of other rat lines and strains classically reported in the literature, the fact that this pattern more closely mimics the human condition, supports the use of the P rat as an animal model of alcoholism. In addition, it justifies the examination of ethanol drinking patterns in other high alcohol-consuming rat lines, so that more analogous models of the human condition can be developed.
Much like the P rat, the replicate high-alcohol-drinking (HAD) rat lines (i.e., HAD-1 and HAD-2) have been selectively bred to voluntarily prefer a 10% ethanol solution over water. While both replicate HAD lines were selectively bred from the same foundation stock using the same criteria (Murphy et al., 2002), there are some quantitative and qualitative differences in ethanol drinking between these two lines under free-choice (Bell et al., 2004), operant (Files et al., 1998; Samson et al., 1998) and alcohol relapse (Rodd et al., 2009; Bell et al., 2008; Oster et al., 2006; Rodd-Henricks et al., 2000) conditions. For instance, adult HAD-1 rats, in comparison to HAD-2 rats, have been shown to consume more ethanol, and display a greater ethanol over water preference ratio in a multiple concentration, free-choice drinking procedure (Bell et al., 2004). In this study, no differences were found between males and females in either replicate line. Subsequent multiple concentration ethanol drinking studies have replicated no sex differences in ethanol consumption in both lines of HAD rats (Bell et al., 2008). In another study using a two-bottle free-choice procedure, there was no difference in ethanol consumption between HAD-1 and HAD-2 male rats (Rodd-Henricks et al., 2000). When looking at operant behavior, studies have shown that HAD-2 rats had a greater number of ethanol responses and consumed a larger amount of ethanol per day than HAD-1 rats (Files et al., 1998; Samson et al., 1998; Oster et al., 2006).
The aims of the present study were to determine the pattern of ethanol consumption and licking behavior seen in adolescent and adult, male and female, HAD-1 and HAD-2 rats during different stages of adolescence and adulthood. Based on previous findings (Bell et al., 2004, 2008), our first hypothesis was that there would be no differences in ethanol consumption between the sexes in both HAD lines. Our second hypothesis was that there would be no difference between the lines on amount of ethanol consumed. This is based on the observation that while the HAD lines differ significantly from each other when given access to ethanol under free-access to multiple ethanol concentration procedures, HAD-1>HAD-2 (Bell et al., 2004) or operant procedures, HAD-2>HAD-1 (Files et al., 1998; Samson et al., 1998; Oster et al., 2006); there was no difference between the lines when given access using a simple two-bottle-choice procedure (Rodd-Henricks et al., 2000), which is the procedure used in the present study. Finally, our main hypothesis was that adolescent HAD rats would display higher ethanol consumption compared with their adult counterparts. This would parallel the general finding that adolescent rodents drink more alcohol than adult rodents.
2. Materials and methods
2.1. Animals
Ethanol-naïve male and female HAD-1 and HAD-2 rats (n = 10–15/age/sex), HAD-1 from the S36–S38 generations and HAD-2 from the S33–S35 generations, were obtained from the Indiana University School of Medicine (Indianapolis, IN, USA) breeding colonies. Adolescent rats were between postnatal days (PND) 21 and 24 of age, when delivered. All rats were double-housed by sex and age. At least four litters were represented in each condition, to limit litter effects. The vivarium was maintained on a 12/12 h light/dark cycle (lights on at 0700 h) with temperature (21 °C) and humidity (50%) controlled. Animals used in these procedures were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine (Indianapolis, IN, USA) and are in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, National Institutes of Health, 2002) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council, 2003).
2.2. Procedures
On PND 28 ± 1 day, for adolescents, or PND 88 ± 1 day, for adults, the animals were single-housed in hanging wire-mesh cages with water and food freely available throughout the experiment. Two days later all rats were given free-choice access to 15% [volume/volume (v/v)] ethanol as well. The ethanol and water bottles were standard glass bottles holding approximately 300 ml of fluid, with a stopper (no. 10) holding an angled (~135°) stainless steel sipper tube. Because the bottles were outside the cage and the sipper tube protruded into the metal cage, the sipper tubes were insulated with black shrink wrap except for 1.5 cm at the tip and another 1.5 cm as the sipper tube exited the bottle through the no. 10 stopper. Solutions were changed twice a week, and bottles were changed every 2 weeks. Starting on the first day of ethanol access, body weight, and water and ethanol bottle weights were obtained using a Sartorius Balance BP 6100 and Sartorius Interface V24/V28-RS232C(−S)/423 (Sartorius Instruments: McGaw Park, IL, USA) and recorded by a personal computer program (Software Wedge, Professional Edition v 5.0 for DOS, Sartorius Instruments: McGaw Park, IL, USA). Weights were recorded at least 5 days per week. All weights were obtained during the light cycle (1100–1300 h). Values for days when weights were not measured were taken as the average of the weights taken on the days preceding and following the missing data point. Number of licks on the water, or ethanol bottles, was obtained with a “Lickometer” (LabLinc V System) set-up from Coulbourn Instruments (Allentown, PA, USA). Essentially, for each bottle (water or ethanol), an electrical lead was attached to the sipper tube (outside of the stainless steel cage) and another electrical lead was attached to the cage rack (ground). Therefore, when the animal licked the sipper tube tip a circuit was closed. Closures of the circuit were summed and recorded every 6 min from 1300 h through 1100 h the next day by a personal computer program (DATAQ software, Coulbourn Instruments, Allentown, PA, USA).
2.3. Statistical analyses across weeks
From PND 33 through 60, for adolescents, and PND 93 through 120, for adults, weekly averages for body weight (g), ethanol (g of ethanol/kg body weight/day) and water (ml/kg/day) consumption, and ethanol (ml/day) to total fluid (ml/day) preference ratios were computed and evaluated for differences between the lines, sexes and age groups across weeks using 2 × 2 × 2 × 4 (line by sex by age by week) mixed ANOVAs, with week being the within-subjects variable and line, sex and age group being the between-subjects variables. Additionally, weekly averages for number of licks on the ethanol or water bottles were summed in 2 h blocks from 1300 h through 1100 h the next day and evaluated for differences between the lines, sexes and age groups across 2 h blocks and weeks using 2 × 2 × 2 × 4 × 11 (line by sex by age by week by 2 h blocks) mixed ANOVAs, with week and 2 h blocks being the within-subjects variables and line, sex as well as age factors being the between-subjects variables. Alpha was set at 0.05 for all analyses. Post hoc Fisher's Least Significant Difference (LSD) t-tests followed significant higher-order interactions and main effects.
3. Results
3.1. Body weight, ethanol intake, water intake, and preference ratio across weeks
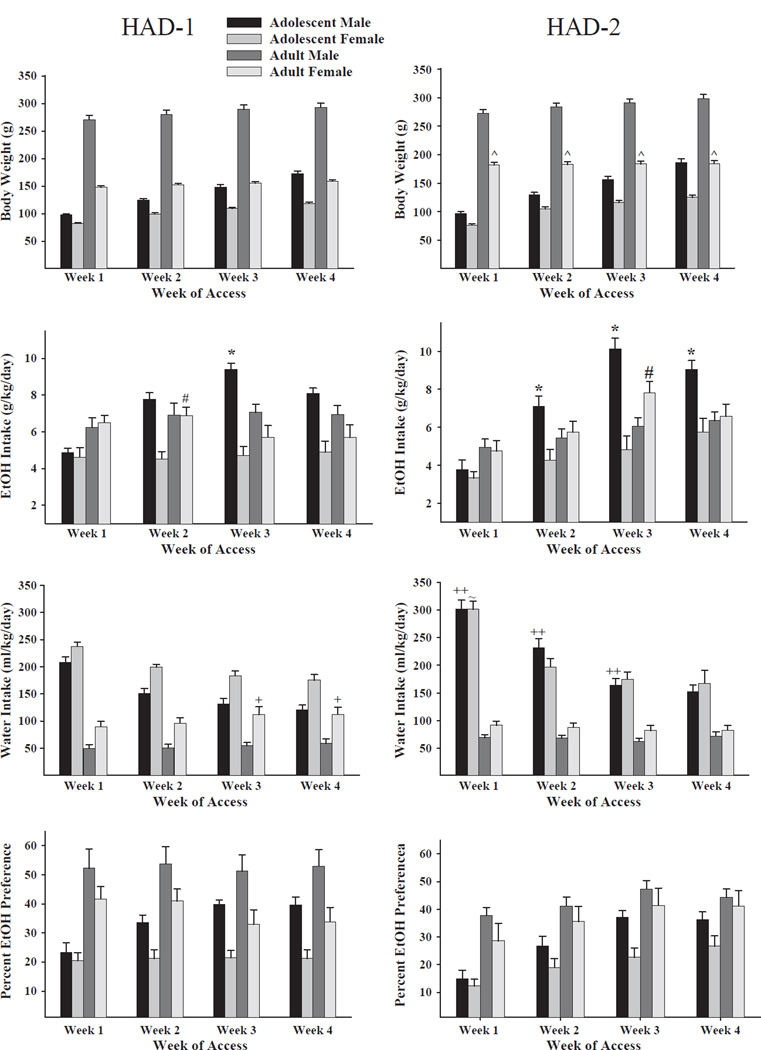
Concerning body weight (g), the omnibus 2 × 2 × 2 × 4 (line by sex by age by week) mixed ANOVA revealed a significant interaction for line by sex by age: [F(1,109) = 11.65, p<0.05]; and significant main effects for line: [F(1,109) = 9.500, p<0.01]; sex: [F(1,109) = 474.921, p<0.0001]; age: [F(1,109) = 918.924, p<0.0001]; and week: [F(3,327) = 1549.067, p<0.0001]; such that adult HAD2 female rats weighed more than adult HAD1 female rats, adult rats weighed more than adolescent rats, male rats weighed more than female rats, and rats gained weight across weeks (Fig. 1, top row).
Fig. 1.
Effects of sex and age of HAD1 and HAD2 rats on body weight (BW) (g), ethanol intake (g/kg/day), water intake (ml/kg/day), and percent ethanol preference across the 4 weeks of ethanol access (n = 13–15/age/sex). All data are representations of the mean ± S.E.M. Adult female HAD-2 rats had a higher BW than adult female HAD-1 rats (^). Adolescent male rats consumed significantly more alcohol than adult male rats; HAD-2 rats during the 2nd through 4th weeks and HAD-1 rats during the third week (*). Adult female rats consumed significantly more alcohol than adolescent female rats; HAD-2 rats during the 4th week and HAD-1 rats during the 2nd week (#). Adolescent male HAD-2 rats consumed significantly more water than adolescent HAD-1 rats during the first three weeks (++). Adolescent female HAD-2 rats drank significantly more water than adolescent female HAD-1 rats during the 1st week (~). Adult female HAD-1 rats consumed significantly more water than adult female HAD-2 rats during the 3rd and 4th weeks (+). Adolescent male HAD-1 and HAD-2 rats and adolescent female HAD-2 rats increased ethanol preference over weeks.
Regarding ethanol intake (g/kg/day), the omnibus 2 × 2 × 2 × 4 (line by sex by age by week) mixed ANOVA revealed significant interactions for line by sex by age by week: [F(3,327) = 3.459, p = 0.017]; sex by age by week: [F(3,327) = 15.602, p<0.001]; sex by age: [F(1,109) = 23.360, p<0.001]; as well as significant main effects for week: [F(3,327) = 54.463, p<0.001]; and sex: [F(1,109) = 24.785, p <0.001]. In general, there was a subtle effect of line on ethanol intake, as indicated by the significant 4-way and 3-way interactions. The 3-way interaction appears to have been driven by ethanol consumption of adolescent male HAD-1 (3rd week) and HAD-2 (2nd through 4thweeks) rats being substantially higher than all of the other groups, especially their adolescent female counterparts (Fig. 1, second from top panels).
Regarding water intake (ml/kg/day), the omnibus 2 × 2 × 2 × 4 (line by sex by age by week) mixed ANOVA revealed significant interactions for line by sex by age by week: [F(3,327) = 4.266, p<0.01]; strain by sex [F(1,109) = 7.981, p<0.01]; as well as significant main effects for line: [F(1,109) = 5.869, p<0.05]; sex: [F(1,109) = 15.496, p<0.0001]; age: [F(1,109) = 272.196, p<0.0001]; and week: [F(3,327) = 191.924, p<0.001]. Female HAD1 rats consumed more water (ml/kg/day) than male HAD1 rats. HAD2 rats consumed more water than HAD1 rats. Adolescent rats consumed more water than adult rats, and water consumption decreased over weeks. Also, adult female HAD-1 rats consumed more water than adult female HAD-2 rats during the third and fourth weeks (Fig. 1, third from top panels).
As for ethanol preference ratio [(ml of ethanol/total ml of fluid) × 100], the omnibus 2 × 2 × 2 × 4 (line by sex by age by week) mixed ANOVA revealed a significant sex by age by week interaction: [F(3,327) = 2.61, p = 0.05]; and significant main effects for sex: [F(1,109) = 16.75, p<0.001]; age: [F(1,109) = 38.77, p<0.001]; and week: [F(3,327) = 22.87, p<0.001]. In general, adult rats had greater preference ratios for ethanol across weeks than adolescent rats, and preference ratios were greatest for adult male rats (Fig. 1, bottom panels). Adolescent male rats from both lines increased ethanol preference over weeks.
3.2. Ethanol and water consumption pattern (licks/2 h) across weeks
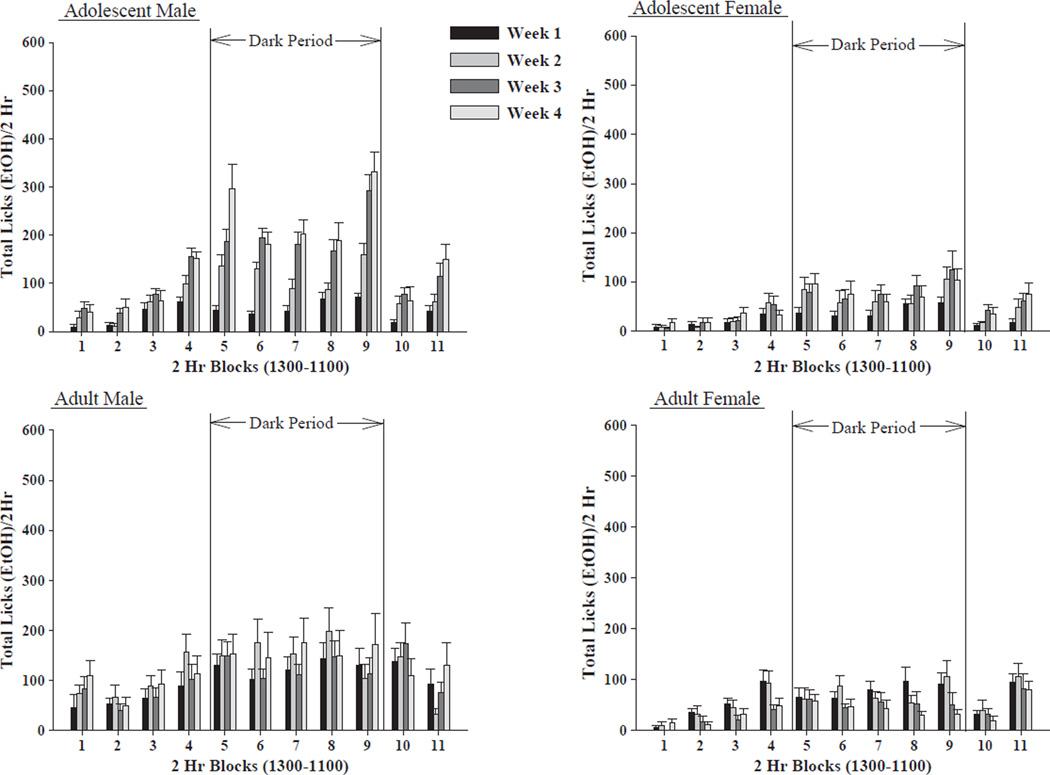
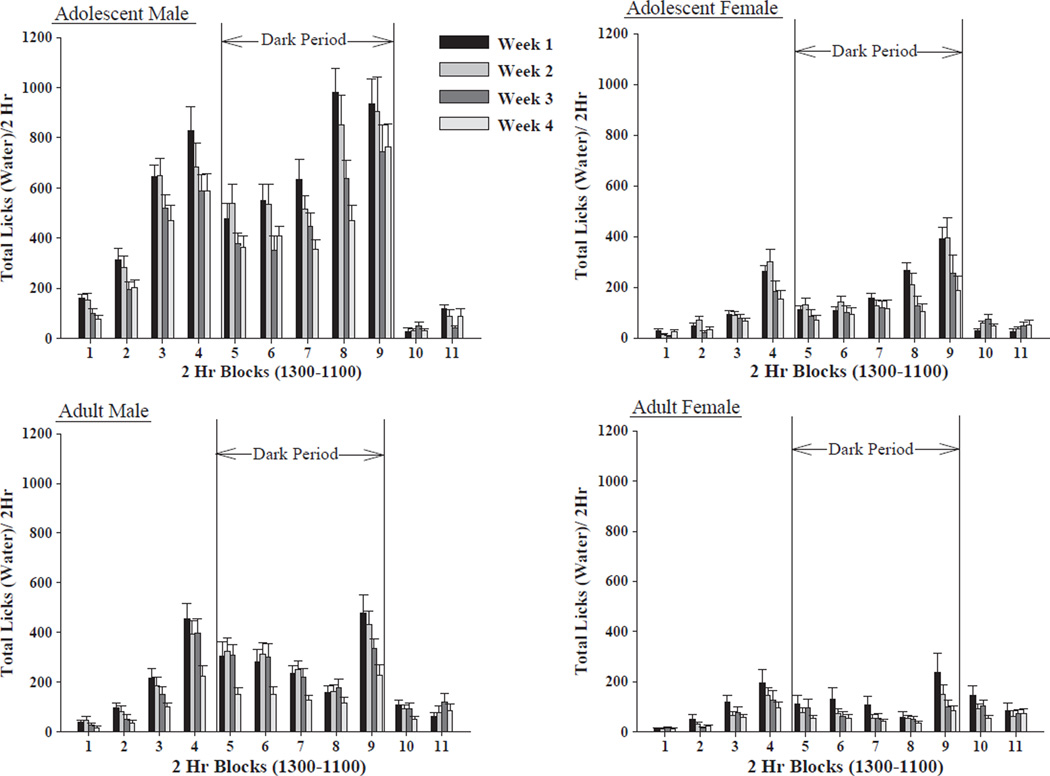
Regarding ethanol consumption pattern, the omnibus 2 × 2 × 2 × 4 × 11 (line by sex by age by week by time)mixed ANOVA revealed a significant 5-way interaction: [F(30,3240) = 2.099, p<0.001]. Thus, each line was analyzed separately. For HAD-1 rats, there was a significant 4-way sex by age by week by time interaction: [F(30,1620) = 1.574, p = 0.025];with significant main effects for sex: [F(1,54) = 24.25, p<0.001];week: [F(3,1620) = 101.33, p<0.001]; and time: [F(10,1620) = 33.625, p<0.001]. Ethanol licking behavior was higher in males than females, and was highest in adolescent male rats. In all rats, ethanol licking behavior was highest during the dark cycle, an effect that was most apparent in the adolescent male. While the adolescent male rats showed an increase in ethanol licking behavior over weeks, the adolescent female rats maintained a steady level of ethanol licking behavior across the second through fourth weeks. While the adult male rats maintained a steady state of ethanol licking behavior over weeks, adult female rats displayed a modest decrease in ethanol licking behavior across weeks (Fig. 2).
Fig. 2.
Ethanol licking behavior in the HAD-1 rat line. Effect of sex of animal (male vs. female HAD-1 rats), age of animal (adolescent vs. adult HAD-1 rats), week of access and time of day on ethanol (15%, v/v) licking behavior (mean ± S.E.M.) across the 4 weeks of ethanol access (n = 13–15/age/sex). Male animals licked the ethanol bottle sipper tube more often than female animals, with most of the ethanol licking behavior of both adolescent and adult HAD-1 rats taking place during the dark cycle. Adolescent male HAD-1 rats consumed the most ethanol of all four groups, see also Fig. 1. Additionally, increases in ethanol licking behavior across weeks were primarily displayed by the adolescent male HAD-1 rats.
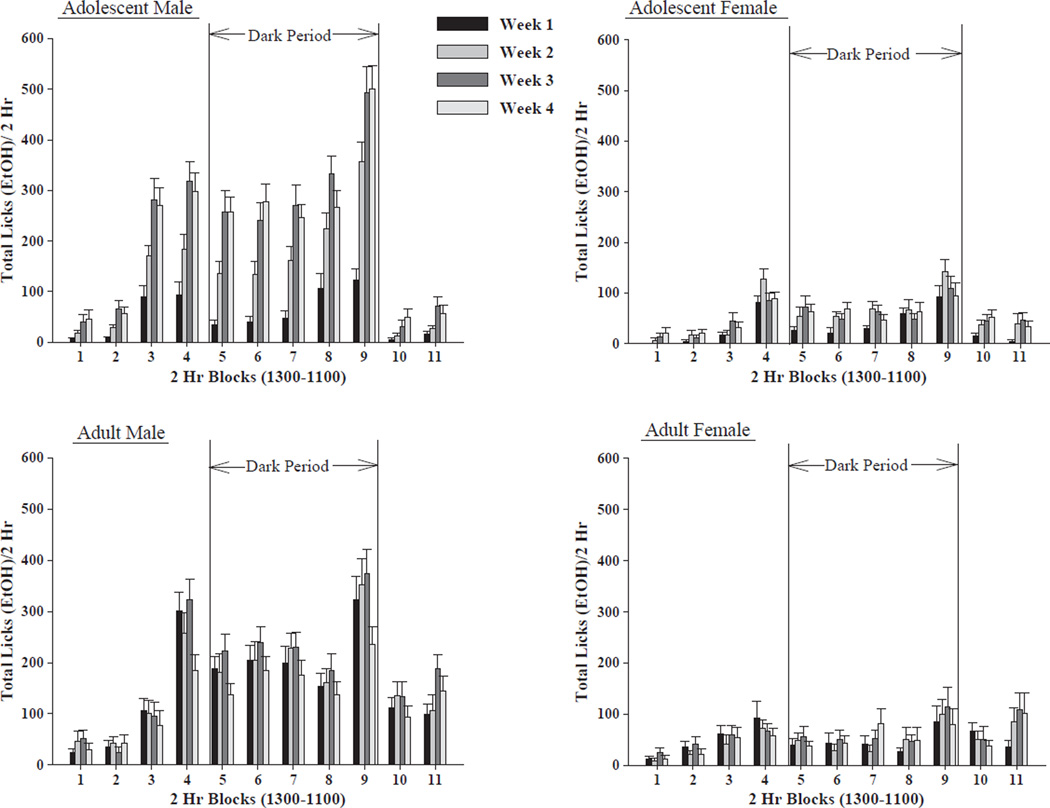
For HAD-2 rats there was a significant 4-way sex by age by week by time interaction: [F(30,1620) = 4.426, p<0.001]; with significant main effects for sex: [F(1,54) = 74.06, p<0.001]; week: [F(3,1620) = 22.457, p<0.001]; and time: [F(10,1620) = 71.027, p<0.001]. Ethanol licking behavior was higher in male rats than female rats, and higher in adolescent male rats than adult male rats. In all rats, ethanol licking behavior was highest during the dark cycle. While the adolescent male rats showed an increase in ethanol licking behavior over weeks, all other groups maintained levels of ethanol licking behavior over weeks. Ethanol licking behavior increased four hours prior to the dark cycle for the adolescent male rats and the adult female rats, with the increase being more pronounced in the adolescent male rats. Ethanol licking behavior increased two hours prior to the dark cycle for the adolescent female and the adult male rats as well. During the four hours after the dark cycle, ethanol licking behavior was higher in the adult male rats than the adolescent male rats (Fig. 3).
Fig. 3.
Ethanol licking behavior in the HAD-2 rat line. Effect of sex of animal (male vs. female HAD-2 rats), age of animal (adolescent vs. adult HAD-2 rats), week of access and time of day on ethanol (15%, v/v) licking behavior (mean ± S.E.M.) across the 4 weeks of ethanol access (n = 13–15/age/sex). Male animals licked the ethanol bottle sipper tube more often than female animals, with most of the ethanol licking behavior of both adolescent and adult HAD-2 rats taking place during the dark cycle. Adolescent male HAD-2 rats showed the most ethanol licking behavior of all four groups. Additionally, increases in ethanol licking behavior across weeks were primarily displayed by the adolescent male HAD-2 rats.
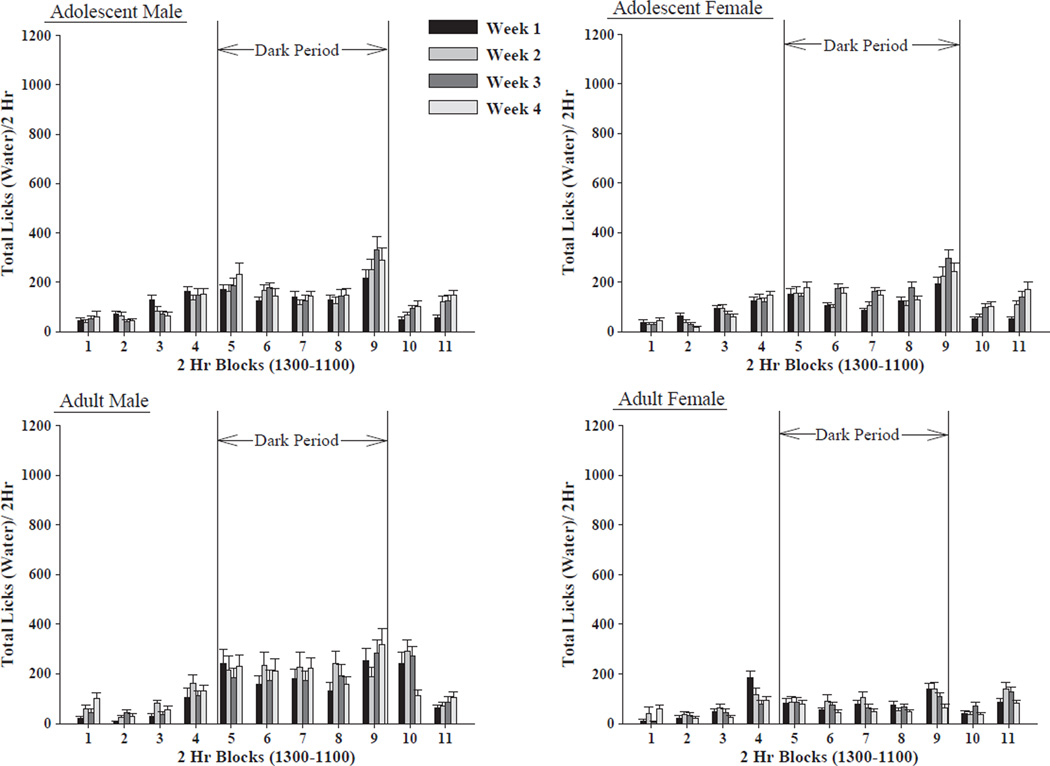
Regarding patterns of water consumption, the omnibus 2 × 2 × 2 × 4 × 11 (line by sex by age by week by time) mixed ANOVA revealed a significant 5-way interaction: [F(30,3240) = 3.085, p<0.001]. Again, each line was examined separately. For HAD-1 rats, there was a significant 4-way sex by age by week by time interaction: [F(30,1620) = 3.312, p = 0.001]; with significant main effects for sex: [F(1,54) = 8.241, p<0.01]; week: [F(3,1620) = 3.217, p<0.05]; and time: [F(10,1620) = 55.529, p<0.001]. Adult female rats showed the least amount of water licking behavior when compared to the adolescent male and female rats, as well as the adult male rats. Water licking behavior was highest during the dark period in the latter three groups.
For HAD-2 rats there was a significant 4-way sex by age by week by time interaction: [F(30,1620) = 2.330, p<0.001]; with significant main effects for sex: [F(1,54) = 62.095, p<0.001]; age: [F(1,54) = 27.41, p<0.001]; week: [F(3,1620) = 27.464, p<0.001]; and time: [F(10,1620) = 102.422, p<0.001]. Adolescent and adult male rats displayed more licking behavior than the adolescent and adult female rats. Adolescent male rats showed more licking behavior than adult male rats, and adolescent female rats showed more licking behavior than adult female rats. Water licking behavior was highest during the dark period in all four groups. In adolescent male rats, water licking behavior increased four hours prior to the park period. In the other three groups, water licking behavior increased two hours before the dark period. Water licking behavior decreased across weeks in all four groups (Figs. 4–5).
Fig. 4.
Water licking behavior in the HAD-1 rat strain. Effect of sex of animal (male vs. female HAD-1 rats), age of animal (adolescent vs. adult HAD-1 rats), week of access and time of day on water licking behavior (mean ± S.E.M.) across the 4 weeks of ethanol access (n = 13–15/age/sex). Adult female HAD-1 rats displayed less water licking behavior that the other three groups of rats. Most of the water licking behavior of both adolescent and adult HAD-1 rats took place during the dark cycle.
Fig. 5.
Water licking behavior in the HAD-2 rat strain. Effect of sex of animal (male vs. female HAD-2 rats), age of animal (adolescent vs. adult HAD-2 rats), week of access and time of day on water licking behavior (mean ± S.E.M.) across the 4 weeks of ethanol access (n = 13–15/age/sex). Water licking behavior was higher in the males when compared to the females. Adolescent males showed more water licking behavior than adult males. Decreases in water licking behavior across weeks were displayed by all of the groups, although the magnitude differed among the groups. Most of the water licking behavior of both adolescent and adult HAD-2 rats took place during the dark cycle.
4. Discussion
In the present study, the pattern of ethanol and water consumption and licking behavior seen in adolescent and adult, male and female, HAD-1 and HAD-2 rats during different stages development was examined. The major findings were that (1) adolescent male HAD-1 and HAD-2 rats increased ethanol consumption levels and ethanol licking behavior throughout adolescence, and engaged in the greatest ethanol consumption and licking behaviors of all four age and sex groups; (2) adolescent female HAD-1 and HAD-2 rats consumed the least amount of alcohol of all four age and sex groups, with HAD-1 adolescent females showing no change in alcohol consumption levels throughout adolescence; (3) adult HAD-1 rats did not show any difference in ethanol consumption levels between the sexes; and (4) adult HAD-2 male and female rats differed in the pattern of ethanol consumed over weeks, with adult female rats consuming more ethanol than adult male rats during the third week of adulthood.
Based on previous findings (Bell et al., 2004, 2008), our first hypothesis was that there would be no differences in ethanol consumption between the sexes in adulthood in both HAD lines. This hypothesis was supported by our findings in the HAD-1 line. Also, while there was an effect of sex in the adult HAD-2 line, the effect was transient with adult female HAD-2 rats consuming more than their male counterparts during the 3rd week of access. Our second hypothesis was that there would be no difference in ethanol consumption levels between the HAD lines. This was partially supported by the lack of a main effect of line from the omnibus 2 × 2 × 2 × 4 mixed ANOVA. However, the significant 4-way line by sex by age by week interaction indicated a subtle effect of line. This appeared to be driven by adolescent and adult HAD-2 rats drinking less ethanol during the 1stweek than theirHAD-1 counterparts, with this effect continuing into the 2nd week for adult HAD-2 rats.
When examining sex-of-animal effects between the two lines during adulthood, HAD-1 male and female rats did not differ in the amount of ethanol consumed per kg, with no change in ethanol consumption levels or licking behavior over time. While ethanol licking behavior was higher in the HAD-1 adult males than females, it should be noted that water licking behavior was also higher in the males than females, even though HAD-1 adult male rats consumed less water than their female counterparts. This indicates that the amount of fluid consumed by the male per lick is less than the female, at least for water, and may explain the seemingly contradictory results between the consumption data and the licking data. For the HAD-2 rats, male and female adult rats did not differ from one another with the exception of the third week, when the adult female rats consumed more alcohol than the adult male rats. Also, while ethanol consumption levels remained constant in the adult male rat, levels increased in the female rat, peaking during week three. Similar to our observations of HAD-1 rats, while ethanol licking behavior was higher in adult HAD-2 males than females, it should be noted that water licking behavior was also higher in the males than females, even though the adult HAD-2 male and female rats did not differ in the amount of water consumed. This indicates that, much like the HAD-1 rat, in the HAD-2 line, the amount of fluid consumed per lick by the male is less than that of the female. Again, this may explain the seemingly contradictory results between the consumption data and the licking data. Overall, the data indicate that HAD rats mimic, at least in some aspects, the human condition with male HAD rats consuming more alcohol than female HAD rats during the transition from adolescence to adulthood (reviewed in Schulte et al., 2009; SAMHSA, 2008; Kessler et al., 1994; Grant and Dawson, 1997). However, adult male HAD rats did not differ in their ethanol consumption from their female counterparts during adulthood when access was initiated during adulthood. Future research is needed to determine whether this overall lack of a sex-of-animal effect would be maintained when the ethanol drinking behavior of adolescent HAD rats is followed well into adulthood.
The lack of sex differences in ethanol consumption behavior of adult HAD-1 rats and the modest difference seen in HAD-2 rats suggest that the use of adult HAD rats in examining sex-differences in alcohol consumption during adulthood may not be the most productive approach, at least as a model of human behavior. Other models that have shown adult male rats consume more ethanol than adult female rats, as was seen in P rats (Bell et al., 2006), might be a better approach. The finding that adult female HAD-2 rats consumed more ethanol than their male counterparts, at least during the third week of ethanol access parallels previous work indicating greater intake by adult female rodents than male rodents. Adult female rodents generally consume more ethanol, in grams per kilogram of body weight, than their male counterparts (Adams, 1995; Juárez and Barrios de Tomasi, 1999; Lancaster and Spiegel, 1992; Li and Lumeng, 1984; Moore et al., 2010; Truxell et al., 2007; Vetter-O'Hagen et al., 2009). This sex-specific effect, although modest, has also been found in peri-adolescent and post-weaning selectively bred rats (Bell et al., 2003, 2004; McKinzie et al., 1998). Findings on ethanol intake by Wistar rats using a limited access schedule revealed that adolescent and adult female Wistar rats consistently drank more ethanol than their male counterparts (Walker et al., 2008). In a study examining drinking-in-the-dark (DID) ethanol intake of adolescent or adult C57BL/6J and DBA/2J mice, Moore et al. (2010) reported that, in general, female mice during both stages of development consumed more ethanol than their male counterparts. This suggests that these two inbred mouse strains do not display the sex by age interaction regarding ethanol intake reported in the present study and previously reported in Sprague–Dawley rats (Truxell et al., 2007; Vetter-O'Hagen et al., 2009).
It should be noted that Vetter-O'Hagen et al. (2009) make the important observation that sex-differences in ethanol intake (female rodents consuming more than male rodents in g/kg) may be related to the concentration of ethanol presented. These authors discuss this hypothesis in light of findings from Cailhol and Mormede (2001, 2002) whose findings suggest that sex-differences are more robust when consuming 10% ethanol than at lesser concentrations. Vetter-O'Hagen et al. (2009) found that female Sprague–Dawley rats consumed significantly more ethanol than their male counterparts when the concentration was increased from 6% to 10%. These findings parallel other reports of sex-differences, with female rodents consuming more than male rodents, when a 10% ethanol solution was used (Adams, 1995; Blanchard et al., 1993; Blizard et al., 2004; Chester et al., 2006; Doremus et al., 2005; Li and Lumeng, 1984). However, there are reports of female rodents consuming more ethanol than male rodents when the concentration was less than 10% or was increased from a lesser concentration to 10% (Juárez and Barrios de Tomasi, 1999; Lancaster et al., 1996; Lancaster and Spiegel, 1992; Vetter-O'Hagen et al., 2009; Walker et al., 2008) as well as reports of this effect with concentrations greater than 10% (Moore et al., 2010; Truxell et al., 2007). Regarding these last two studies the former study was conducted in mice and both studies were longitudinal studies examining adolescent to adult developmental changes. Therefore, the use of a 15% ethanol concentration in the present study may have masked the usual female greater than male sex-difference in ethanol intake. Another possibility for the lack of the commonly seen sex-difference may be due to alterations, relative to outbred lines, in the hypothalamic–pituitary–gonadal axis between the sexes of HAD rats, or by extension alterations in circulating levels of neurosteroids. Further research is needed to address these possibilities.
A major finding of the present study is that the adolescent HAD-1 and HAD-2 male rats displayed the highest ethanol consumption and licking behavior, including the most well defined ethanol licking binges, of all age-by-sex groups. Adolescent male rats also showed increasing levels of ethanol consumption throughout adolescent development. These findings match the growing literature indicating this effect in other rodent lines and strains (Doremus et al., 2005; Moore et al., 2010; Truxell et al., 2007; Vetter et al., 2007; Vetter-O'Hagen et al., 2009). In the voluntary ethanol consumption lickometer study carried out by Bell et al. (2006), it was shown that adolescent male P rats increased consumption throughout adolescence. However, these authors reported that the intake of adolescent and adult P rats did not differ significantly. In another study carried out by Doremus et al. (2005), adolescent Sprague–Dawley rats voluntarily consumed more ethanol than adults. However these authors reported that instead of increasing ethanol consumption behavior, ethanol consumption declined over time. Since the present findings indicate that the HAD rat animal model of alcoholism mimics the human condition to a better degree than the P or Sprague–Dawley rat models, at least as it pertains to adolescent vs. adult differences, it can be argued that the adolescent male HAD rat can be used effectively as an animal model of binge-drinking and excessive alcohol consumption in adolescent humans.
In contrast to adolescent male HAD rats, adolescent female HAD rats consumed the least ethanol of all groups tested. Adolescent female HAD-1 rats consumed significantly less ethanol than adolescent and adult male HAD-1 rats, except during the 1st week of adolescence when they did not differ. In other studies, it has been found that adolescent female Sprague–Dawley rats consumed less ethanol than their adolescent male counterparts (Truxell et al., 2007; Vetter-O'Hagen et al., 2009). Overall, the results indicate that the female adolescent Sprague Dawley and HAD rats differ from female adolescent humans, in that unlike the human condition (discussed below) these adolescent female rats consume significantly less alcohol than their adolescent male counterparts.
For humans, recent SAMHSA (2008) data indicate that female and male adolescents (12–17 years) report remarkably similar rates for current drinking, 16.0% and 15.9% respectively. Additionally, Johnston et al. (2008) report that differences in binge drinking between adolescent males and females during 8th grade (10.4% vs. 10.0% respectively) and 10th grade (23.4% vs 20.4% respectively) are negligible. Differences between the sexes only begin to emerge during the 12th grade, when more 12th grade boys than girls report binge drinking at 30.7% vs. 21.5% respectively (Johnston et al., 2008). The findings from the male and female HAD rats differ somewhat from the human condition, in that male rats consumed more ethanol than female rats immediately after early adolescence. While the adult HAD rats did not display a sex-of-animal difference, the present study did not follow the ethanol drinking behavior of the adolescent-exposed animals well into adulthood, which may have revealed the presence of sex differences. One other difference that should be noted is that HAD-1 female adolescent rats, unlike female adolescent humans, did not increase ethanol consumption levels over adolescence. Such differences in ethanol consumption behavior between the adolescent rat and adolescent human must be clarified before determining the best animal model of alcohol use disorders for a particular developmental period.
A major hypothesis of this study was that HAD-1 and HAD-2 rat strains would mimic the human condition in two ways. Adolescent HAD rats would increase ethanol consumption over time with rats in late adolescence consuming more ethanol than adults. However, this was only true for adolescent male HAD rats. Our hypothesis based on the human literature was that no difference would be found between adolescent male and female rats until the later stages of adolescence, at which point the male rats would show a higher level of ethanol consumption than female rats. While the HAD rats were similar to the human condition in that adolescent males consumed more ethanol than adolescent females, they differed from the human condition in that HAD rat sex differences began to differ in ethanol consumption levels early in adolescence. This latter effect differs from the human condition where males do not consume more ethanol than females until late in adolescence.
In conclusion, the extensive data provided from this study reveals subtle, and not so subtle, differences in the drinking patterns of adolescent and adult, male and female, HAD-1 and HAD-2 rats. These results provide an impetus to conduct more adolescent alcohol research in the HAD lines of rats. Understanding why adolescent male HAD-1 and HAD-2 rats displayed the highest levels of ethanol consumption, binge drinking, and increased ethanol consumption over time compared to the other groups tested may help us to better address alcohol use disorders among male adolescents. Further research is needed to help elucidate the neurobiological mechanisms that mediate developmental and sex differences in ethanol intake.
Acknowledgments
This work was supported in part by NIH grants AA07611, AA07462, AA10256 and AA13522 (INIA-West).
Footnotes
Conflicts of interest
None of the authors have real or perceived conflicts of interest associated with this work.
References
- Adams N. Sex differences and the effects of tail pinch on ethanol drinking in Maudsley rats. Alcohol. 1995;12:463–468. doi: 10.1016/0741-8329(95)00032-m. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Hsu CC, Lumeng L, Li TK, Murphy JM, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on ethanol intake by periadolescent high-alcohol-drinking rats. Alcohol. 2004;33:107–185. doi: 10.1016/j.alcohol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, et al. Daily patterns of ethanol drinking in periadolescent and adult alcohol preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Schultz JA, Peper CL, Lumeng L, Murphy JM, McBride WJ. Effects of short deprivation and re-exposure intervals on the ethanol drinking behavior of selectively bred high alcohol-consuming rats. Alcohol. 2008;42:407–416. doi: 10.1016/j.alcohol.2008.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63:91–99. [PubMed] [Google Scholar]
- Chester JA, Barrenha GDP, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behavior in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcohol Clin Exp Res. 2006;30:1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Denning CE, Marvin S. Comparison of alcohol-preferring and non-preferring selectively bred rat lines. II. Operant self-administration in a continuous-access situation. Alcohol Clin Exp Res. 1998;22:2147–2158. [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: overview of key findings, 2007. Bethesda, MD: National Institute on Drug Abuse; 2008. (NIH publication no. 08-6418). [Google Scholar]
- Juárez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19(1):15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliot JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague–Dawley rats. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Li T-K, Lumeng L. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res. 1984;8:485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. Underage drinking: a developmental framework. Pediatrics. 2008;121:S235–S251. doi: 10.1542/peds.2007-2243A. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res. 1998;22:1584–1590. [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón LC, Boehm SL., II Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Office of Laboratory Animal Welfare. Public health service policy on humane care and use of laboratory animals. Bethesda, MD: National Institutes of Health; 2002. [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, et al. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol. 2006;38:155–164. doi: 10.1016/j.alcohol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, McBride WJ. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of high-alcohol-drinking (HAD) rats. Addict Biol. 2009;14:152–164. doi: 10.1111/j.1369-1600.2008.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li T-K. The expression of an alcohol deprivation effect in high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000;24:747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats. I. Periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C, Marvin S. Comparison of alcohol-preferring and non-preferring selectively bred rat lines. I. Ethanol initiation and limited access operant self-administration. Alcohol Clin Exp Res. 1998;22:2133–2146. [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–31. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2008. (DHHS publication no. SMA 08-4343, NSDUH series H-34). [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: prevalence, symptom profiles and correlates. Drug Alcohol Depend. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]