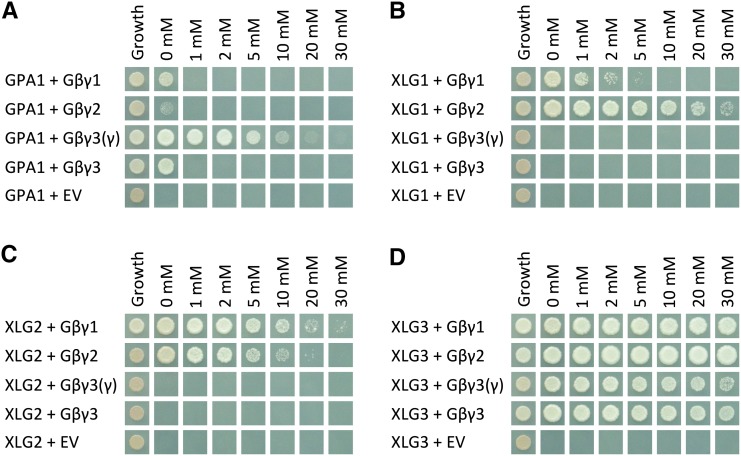
Figure 1.
Yeast three-hybrid assays demonstrate the specificity of GPA1 for AGB1/AGG3, XLG1 and XLG2 for AGB1/AGG1 and AGB1/AGG2, and XLG3 for all three Gβγ dimers. Yeast three-hybrid assays tested the interactions between GPA1 (A), XLG1 (B), XLG2 (C), and XLG3 (D; GAL4 activation domain fusions) with the AGB1/AGG1 (Gβγ1), AGB1/AGG2 (Gβγ2), and AGB1/AGG3 (Gβγ3) Gβγ dimers (Gγ fused to the GAL4 binding domain, with Gβ [AGB1] as the bridge protein). A truncated γ3(γ) was also tested, consisting of only the Gγ-like domain of AGG3 (residues 1–112), lacking the implicated transmembrane domain. Yeast growth on synthetic complete (SC)-Trp-Leu confirmed transformation and cell viability. Interactions were assayed on SC-Trp-Leu-Met-His supplemented with the indicated concentrations of 3-AT (0–30 mm). EV, Empty vector.