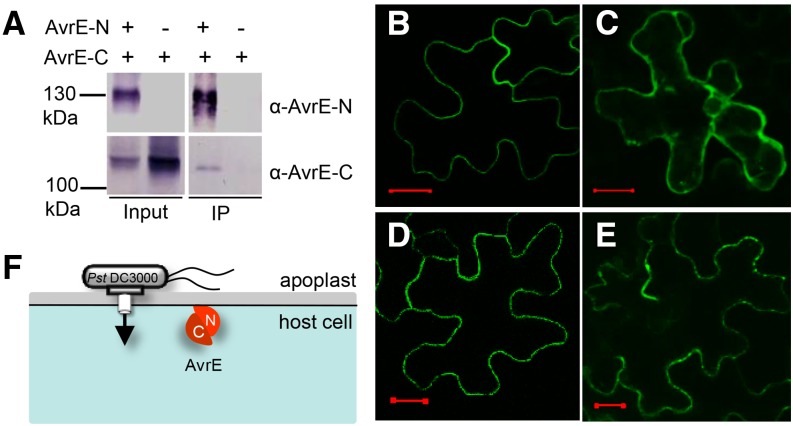
Figure 4.
The two domains of AvrE physically interact, and the N-terminal domain contains a PM localization signal. A, Coimmunoprecipitation shows that AvrE-N and AvrE-C interact in N. benthamiana. The affinity-purified AvrE-N antibody was used to pull down AvrE-N. AvrE-N and AvrE-C were detected by AvrE-N and AvrE-C antibodies, respectively. IP, Immunoprecipitate. B to E, Confocal images of GFP:AvrE-N in N. benthamiana leaf cells (B), GFP:AvrE-C in N. benthamiana leaf cells (z-stack; C), GFP:AvrE-N coexpressed with AvrE-C in N. benthamiana leaf cells (D), and GFP:AvrE-C protein coexpressed with AvrE-N in N. benthamiana leaf cells (z-stack; E). Expression of GFP:AvrE-N and GFP:AvrE-C was under the control of the cauliflower mosaic virus 35S promoter (pSITEII vector). A. tumefaciens containing GFP:avrE-N/C and DEX:avrE-C/N constructs was adjusted to OD600 = 0.1 and mixed at a ratio of 1:2 (GFP:avrE-N/C versus DEX:avrE-C/N) for infiltration. Twenty-four hours after A. tumefaciens infiltration, 10 µm DEX was sprayed to induce the expression of untagged AvrE-N and AvrE-C proteins. Confocal images were collected 5 h after DEX treatment. Bars = 20 µm. F, A simplified model showing the two physically interacting domains of AvrE and their localization in the plant cell. C, AvrE-C; N, AvrE-N.