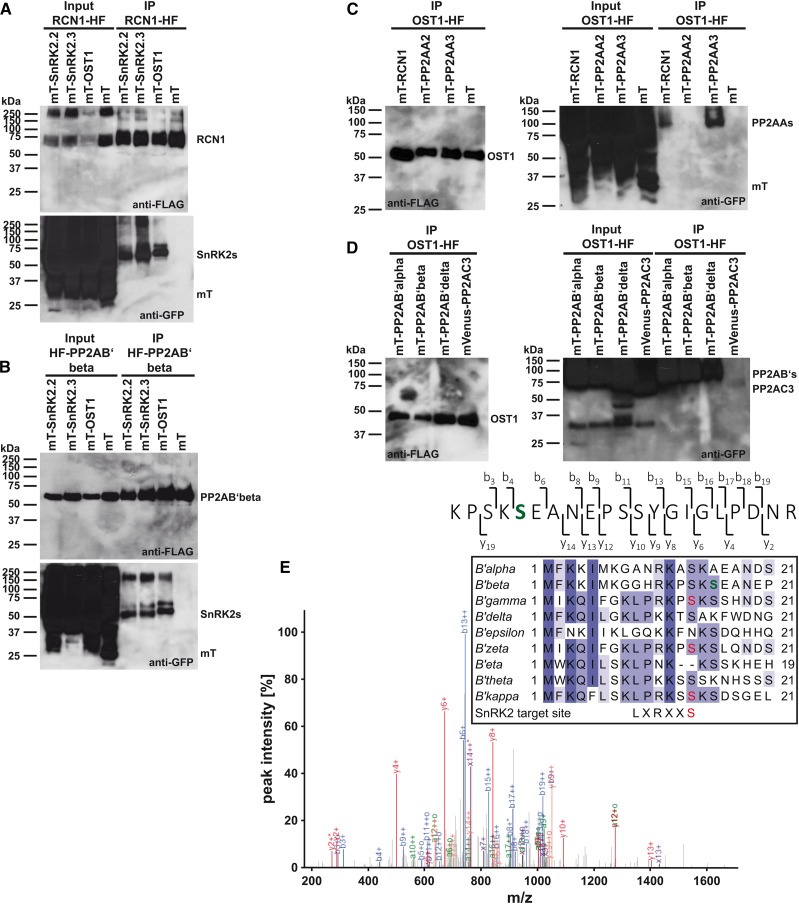
Figure 3.
SnRK2-type protein kinases interact with PP2A-type protein phosphatase regulatory subunits in co-IP analyses. A, Co-IP analyses of the regulatory PP2AA-subunit RCN1-HF with (mT)urquoise-SnRK2s and mT. B, Co-IP analyses of the regulatory PP2AB′-subunit HF-PP2AB′beta with mT-SnRK2s and mT. C, Co-IP analyses of OST1-HF with mT-PP2AA regulatory A subunits and mT. D, Co-IP analyses of OST1-HF with mT-PP2AB′ regulatory B′ subunits and mVenus-PP2AC3 catalytic C subunit. A to D, Western blots of HF-tagged proteins (anti-FLAG) and mT- or mVenus-tagged proteins (anti-GFP) after coexpression in N. benthamiana (input) and anti-FLAG immunoprecipitation (IP) of HF-tagged proteins. E, MS/MS spectrum of an HF-PP2AB′beta phosphopeptide after copurification with mVenus-OST1. The high-intensity fragment ion peaks from b6 onward indicate that one of the first two Ser residues is phosphorylated. Fragment ion b3, observed as low-intensity peak, localizes the site of modification to S16 (green). E, inset, MuscleWS alignment of PP2AB′ subunits. Conserved amino acids are highlighted by different tones of purple. PP2AB′beta S16 (green) is conserved in six of nine PP2AB′ subunits. PP2AB′gamma, PP2AB′zeta, and PP2AB′kappa harbor an SnRK2-type protein kinase consensus target site (red; Vlad et al., 2008; Sirichandra et al., 2010).