Recent insights into the role of ethylene in regulating plant growth and development highlight interactions with other hormones.
Abstract
Ethylene is a gaseous plant hormone that most likely became a functional hormone during the evolution of charophyte green algae, prior to land colonization. From this ancient origin, ethylene evolved into an important growth regulator that is essential for myriad plant developmental processes. In vegetative growth, ethylene appears to have a dual role, stimulating and inhibiting growth, depending on the species, tissue, and cell type, developmental stage, hormonal status, and environmental conditions. Moreover, ethylene signaling and response are part of an intricate network in cross talk with internal and external cues. Besides being a crucial factor in the growth control of roots and shoots, ethylene can promote flowering, fruit ripening and abscission, as well as leaf and petal senescence and abscission and, hence, plays a role in virtually every phase of plant life. Last but not least, together with jasmonates, salicylate, and abscisic acid, ethylene is important in steering stress responses.
This Update provides recent insights into the role of ethylene on vegetative growth, both at the cellular and the whole-plant levels, with special attention to hormonal cross talk. Due to space restrictions, this Update is mainly focused on Arabidopsis (Arabidopsis thaliana).
CELLULAR RESPONSES: ETHYLENE IN THE CONTROL OF CELL DIVISION, CELL ELONGATION, AND CELL DEATH
Ethylene and Cell Division
Few reports have addressed the role of ethylene on cell division, revealing opposite actions on the cell cycle, depending on tissue type and internal and external cues. Ethylene stimulates cell division in the subepidermal layers during apical hook development, probably acting in cooperation with auxins (Raz and Koornneef, 2001). The role of ethylene on cell division in roots is somewhat conflicting. Ethylene does not change the expression pattern of the CYCLIN-DEPENDENT PROTEIN KINASE B1;1 (CYCB1;1)-GUS reporter, indicating that it does not affect mitotic activity in the root (Růzicka et al., 2007). Yet, it was shown that ethylene modulates cell division in the stem cell niche of the quiescent center (QC), resulting in supernumerary QC cells that will develop in extra columella layers in the root cap (Ortega-Martínez et al., 2007). Furthermore, root apical meristem (RAM) size and cell number were shown to be controlled by CULLIN3-type E3 ligases in an ethylene-dependent manner (Thomann et al., 2009). Recently, it was confirmed genetically and pharmacologically that ethylene inhibits cell proliferation of the RAM, resulting in smaller meristems (Street et al., 2015). The action of ethylene on RAM size was shown to be mediated by the ethylene receptors signaling primarily via the canonical ethylene signaling pathway (over CONSTITUTIVE TRIPLE RESPONSE1 [CTR1]-ETHYLENE INSENSITIVE2 [EIN2]-EIN3/ETHYLENE INSENSITIVE3-LIKE [EIL]) and in part via an alternative two-component signaling cascade involving ARABIDOPSIS RESPONSE REGULATOR1 (ARR; a B-type ARR transcription factor; Street et al., 2015). Furthermore, in the control of RAM proliferation, ethylene was found to interact with both cytokinins (CKs) and auxin, the latter likely mediated by the auxin repressor SHORT HYPOCOTYL2 (INDOLE ACETIC ACID3; Street et al., 2015). Ethylene also plays a role during the differentiation of vascular tissue, where it controls cell division rate (Etchells et al., 2012). It was shown that ETHYLENE OVERPRODUCER eto1 and eto2 mutations stimulate vascular cell division and ethylene signaling was required for vascular cell differentiation (Etchells et al., 2012). In leaves, ethylene was shown to cause cell cycle arrest upon mild osmotic stress, through the inhibition of CYCLIN-DEPENDENT KINASE A activity and independently from EIN3 transcriptional control (Skirycz et al., 2011). Ethylene also inhibits the mitotic cell cycle in the abaxial cells of the leaf petiole through the down-regulation of CYCLIN2A;1 expression, partially contributing to the hyponastic response (Polko et al., 2015).
Ethylene and Cell Elongation
Although ethylene is best known for its inhibition of cell elongation both in dark-grown seedlings (as part of the triple response; Bleecker et al., 1988; Guzmán and Ecker, 1990) and in light-grown plants (Rodrigues-Pousada et al., 1993), there are several observations of ethylene-stimulated cell elongation. For example, ethylene induces cell elongation in the hypocotyl of seedlings grown in the light (Smalle et al., 1997) as well as root hair elongation (Pitts et al., 1998) and petiole elongation in certain ecotypes (Millenaar et al., 2005). Cellular elongation is the net result of several processes, including but not limited to the rearrangement of the cytoskeleton, the relaxation of the cell wall, and the uptake of water to establish sufficient turgor pressure. Some of these processes are (at least in part) regulated by ethylene.
Modification of the interior cytoskeleton, mainly via the rearrangement of cortical microtubules (CMT), controls the direction of elongation (Bashline et al., 2014). Cell elongation is facilitated by changing the orientation of the CMT perpendicular to the growth axis (Bashline et al., 2014). Ethylene has been shown to rapidly (within 10 min) affect microtubule orientation in Arabidopsis roots and hypocotyls, likely preventing cell elongation and facilitating radial swelling (Le et al., 2004, 2005). The opposite process has been observed in upper hypocotyl cells when grown in the light (Le et al., 2005). Ethylene can also induce petiole elongation as part of a hyponastic growth response by rearranging the CMT from a longitudinal toward a transverse orientation in the abaxial cells (but not the adaxial cells; Polko et al., 2012). Recently, it was shown that auxins, like ethylene, can also rapidly induce the reorientation of CMT to prevent root and hypocotyl elongation (Chen et al., 2014). However, this was challenged by Baskin (2015), who demonstrated that, upon removal of nearly all CMT by an oryzalin treatment, seedlings still showed a strong inhibition of root growth rate by auxin, suggesting that the auxin-induced inhibition of cell expansion is independent from CMT. It is also very likely that auxin and ethylene signal via cross talk to regulate CMT orientation, although this remains to be investigated.
Cellular elongation also requires cell wall loosening, which is achieved by rearranging the cell wall matrix polymers with the aid of cell wall-remodeling enzymes (Cosgrove, 2000). Four major groups of cell wall-remodeling enzymes control elongation: expansins (EXP), xyloglucan endotransglycolases/hydrolases (XTH), endo-1,4-β-glucanases, and pectin methylesterases (Sasidharan et al., 2011; Bashline et al., 2014). Of these four, mainly EXP and XTH are involved in ethylene-regulated cellular elongation. In Arabidopsis, expansins have been shown to be ethylene regulated during root hair formation (Cho and Cosgrove, 2002) and the submergence-escape elongation response (Polko et al., 2012; Rauf et al., 2013). During these processes, ethylene induces EXP expression and triggers a local, often tissue-specific, elongation response. It has also been shown that ethylene can regulate XTH expression during root hair initiation (Vissenberg et al., 2001) and the submergence-induced hyponastic response of Arabidopsis (Rauf et al., 2013).
Ethylene and Cell Death
At given stages in the life cycle of plants, vegetative development requires programmed cell death (PCD). PCD facilitates organ growth and/or abscission as well as defense reactions against biotic and abiotic stresses (Van Hautegem et al., 2015).
One of the best known examples of PCD is the formation of xylem cells, called xylogenesis (Bollhöner et al., 2012). It was shown that, during the active phase of xylogenesis, the expression of ethylene biosynthesis genes (both 1-aminocyclopropane-1-carboxylic acid [ACC] synthases [ACS] and ACC oxidases [ACO]) and, consequently, ethylene production were increased, stimulating xylem formation (Andersson-Gunnerås et al., 2003; Love et al., 2009; Pesquet and Tuominen, 2011). Ethylene production and ACO protein abundance were also up-regulated in xylem cells during gravitational bending of woody species (Andersson-Gunnerås et al., 2003), resulting in the modification of xylem cell morphology (Love et al., 2009; Ramos and Herrera, 2013), ultimately leading to the formation of tension wood.
Another example of PCD is the formation of aerenchyma, which stimulates gas exchange during hypoxic conditions, typical for semiaquatic species (Drew et al., 2000). Aerenchyma formation was shown to be ethylene regulated in many species, including Arabidopsis (Mühlenbock et al., 2007).
ETHYLENE AND GERMINATION
A complex balance between plant hormones regulates seed dormancy and germination in response to environmental signals. Ethylene biosynthesis, relying on the activities of ACS and ACO, which convert the precursor ACC to ethylene (Yang and Hoffman, 1984), increases during germination, with a peak production upon radicle protrusion (Corbineau et al., 2014). Although ACS is usually the rate-limiting enzyme, ACO activity plays a crucial role in seed germination. ACO1 and ACO2 are the most important ACOs for ethylene biosynthesis in the Arabidopsis seed (Linkies et al., 2009). Ethylene promotes the germination of primary and secondary dormant seeds and even of nondormant seeds under unfavorable environmental conditions (Corbineau et al., 2014). Consistently, gain-of-function mutations in ETHYLENE RESPONSE1 (ETR1) and loss-of-function mutations of EIN2, which decrease the ethylene response, increased dormancy and postponed germination, whereas mutations in ETO1 and CTR1, which increase ethylene production and response, respectively, reduced dormancy and accelerated germination (Beaudoin et al., 2000; Ghassemian et al., 2000; Chiwocha et al., 2005; Subbiah and Reddy, 2010). A recent study by Wilson et al. (2014b) indicated that, under salt stress, the ethylene receptors ETR1, ETR2, and EIN4 have contrasting functions during Arabidopsis seed germination. Loss of ETR2 delayed germination, while loss of ETR1 and EIN4 advanced germination. However, the involvement of ethylene in the regulation of germination under salt stress by ETR1 and ETR2 appeared to be minor. A difference in ethylene production or sensitivity was not accountable for the contrasting effect on germination upon the loss of ETR1 and ETR2. Furthermore, another study by Wilson et al. (2014a) demonstrated that light, an important trigger of germination, affects the function of ETR1 in germination. In etr1 loss-of-function alleles, germination was promoted in darkness and under far-red light, although not under other light conditions. The latter result and the genetic interaction of ETR1 with PHYTOCHROME A (PHYA) and PHYB suggest that the regulation of germination by ETR1 is mediated by PHYA and PHYB under far-red light. Nevertheless, under white light and in darkness, ETR1 may affect germination independent of PHY signaling. Consistent with Wilson et al. (2014b), ETR1 and ETR2 also had contrasting functions under far-red light, and the involvement of ethylene in the regulation of seed germination by ETR1 again appeared to be minor (Wilson et al., 2014a).
Apart from plant hormones, a role for reactive oxygen species (ROS) in the regulation of seed germination emerged. Recently, the accumulation ROS concomitant with radicle protrusion was shown to be required for seed germination (Liu et al., 2010; Leymarie et al., 2012). Ethylene and ROS interplay is involved in the regulation of cell elongation (El-Maarouf-Bouteau et al., 2014), possibly through cell wall remodeling (Linkies and Leubner-Metzger, 2012); however, this needs to be confirmed for Arabidopsis. Increasing evidence in various plant species, including Arabidopsis, suggests that protein modifications represent a putative mechanism for the cross talk between ethylene and the ROS nitric oxide in the regulation of germination (Arc et al., 2013).
Ethylene Cross Talk during Germination
Seed dormancy and germination are regulated by a complex cross talk of multiple plant hormones. The importance of abscisic acid (ABA) and GAs in the regulation of seed germination has long been established. ABA initiates and maintains seed dormancy, whereas GAs release dormancy and initiate seed germination (Arc et al., 2013; Corbineau et al., 2014; Miransari and Smith, 2014). However, brassinosteroids (BRs), auxins, CKs, and jasmonates (JAs) are also involved in the regulation of seed germination (Linkies and Leubner-Metzger, 2012; Miransari and Smith, 2014). Nevertheless, a possible cross talk with ethylene has not yet been established. Ethylene works antagonistically with ABA but synergistically with GAs in the regulation of Arabidopsis seed germination. The activity of ethylene in the regulation of seed dormancy and germination is based on reciprocal effects on both ABA and GA biosynthesis and signaling (Arc et al., 2013; Corbineau et al., 2014). Upon ABA and GA treatment during germination, ethylene biosynthesis is affected through changes in ACO rather than ACS expression. Furthermore, by means of the ethylene biosynthesis mutant aco2, ethylene production by ACO2 has been demonstrated to hinder the ABA-controlled inhibition of endosperm rupture (Linkies et al., 2009; Linkies and Leubner-Metzger, 2012). Apart from ABA biosynthesis, ethylene also affects the sensitivity to ABA in the regulation of germination (Beaudoin et al., 2000; Ghassemian et al., 2000; Cheng et al., 2009). In the eto3 and ctr1 mutants, ABA sensitivity was significantly reduced, while it was significantly enhanced in ethylene-insensitive alleles of etr1, ein2, and ein6 (Subbiah and Reddy, 2010). Wilson et al., (2014b) demonstrated that loss-of-function etr1 and etr2 mutants have reduced and enhanced ABA sensitivity during salt stress, consistent with their faster and delayed germination, respectively. The function of ETR1 and ETR2 during salt stress thus seems to be mediated by ABA rather than ethylene signaling. Moreover, under far-red light, loss of ETR1 was demonstrated to affect ABA and GA biosynthesis and sensitivity during germination (Wilson et al., 2014a).
ETHYLENE AND APICAL HOOK DEVELOPMENT
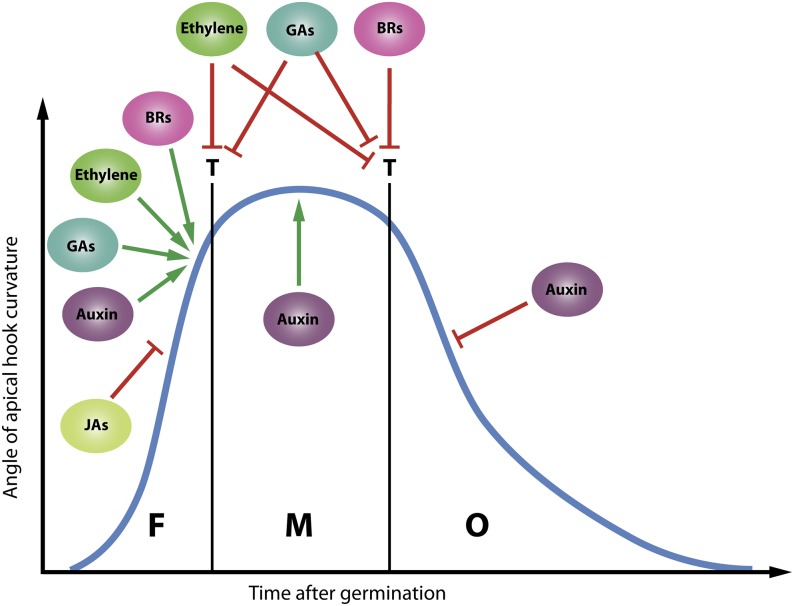
To protect the delicate shoot apical meristem and cotyledons from damage upon soil protrusion after underground germination, the hypocotyl of dicotyledonous seedlings forms a hook-like structure (Darwin and Darwin, 1881; Guzmán and Ecker, 1990). The formation of the apical hook initiates shortly after germination. When the hook is completely closed, it is maintained until exposure to light after emergence from the soil, which heralds its unfolding (Raz and Ecker, 1999). The gradual bending of the hook rests predominantly on differential growth mediated by an auxin gradient, regulated by a complex hormonal cross talk (Fig. 1; Abbas et al., 2013; Mazzella et al., 2014; Žádníková et al., 2015).
Figure 1.
Simplified cross talk diagram on the regulation of the dynamics of apical hook development. Apical hook development proceeds in three distinct phases: formation (F), maintenance (M), and opening (O). Hook formation initiates shortly after germination. After complete formation, the hook is maintained for several hours before opening (the apical hook dynamics shown here are for complete darkness). The formation of the apical hook depends on differential elongation driven by an auxin gradient, which is formed, maintained, and lost concomitant with the three phases of apical hook development. Ethylene plays a central role upstream of auxin. BRs, GAs, and JAs interact with ethylene in the regulation of the three phases of apical hook development and the intermediary transitions (T). Red arrows indicate inhibitory effects, whereas green arrows indicate stimulatory effects.
In dark-grown Arabidopsis seedlings, ethylene elicits an exaggeration of the apical hook, the inhibition of both hypocotyl and root elongation, and radial swelling of the seedling stem. The exaggeration of the hook curvature as part of the triple response (Bleecker et al., 1988; Guzmán and Ecker, 1990) stresses an important function of ethylene in apical hook development and results from a delay in the transition between hook formation and maintenance (Fig. 1; Vandenbussche et al., 2010; Žádníková et al., 2010). Ethylene also regulates the transition between hook maintenance and opening by preventing hook opening (Fig. 1; Vandenbussche et al., 2010; Gallego-Bartolomé et al., 2011; Smet et al., 2014). Ethylene biosynthesis, perception, and signaling are indispensable for a flawless development of the apical hook. Ethylene-insensitive gain-of-function receptor mutants have a defective apical hook, whereas ethylene-hypersensitive alleles exhibit an exaggerated apical hook curvature in the presence of ethylene. The constitutive ethylene response mutant ctr1 exhibits an exaggerated hook in the absence of ethylene (Abbas et al., 2013; Mazzella et al., 2014). Apical hook development is deficient in the ethylene-insensitive mutant ein2 (Guzmán and Ecker, 1990), resulting from a severe inhibition of apical hook formation (Smet et al., 2014). Similarly, loss of function of the transcription factor EIN3 results in a reduced curvature, whereas EIN3 overexpression induces an enhanced apical hook curvature (An et al., 2012a). The ethylene-responsive gene HOOKLESS1 (HLS1), which encodes an N-acetyltransferase, is indispensable for apical hook formation (Guzmán and Ecker, 1990; Lehman et al., 1996). Recently, An et al. (2012a) showed that ethylene activates the transcription of HLS1 through direct binding of the EIN3/EIL1 transcription factors to its promoter.
Ethylene Cross Talk during Apical Hook Development
The differential elongation of the hypocotyl, underlying apical hook development, is driven by an auxin gradient (Friml et al., 2002; Li et al., 2004; Vandenbussche et al., 2010; Žádníková et al., 2010; Gallego-Bartolomé et al., 2011). Auxin is differentially distributed in the apical part, with an accumulation at the concave side exceeding that of the convex side, concomitant with hook formation and maintenance. Hook opening is associated with the fading of the asymmetrical auxin distribution (Fig. 1). The auxin gradient is established by polar auxin transport regulated by a complex hormonal network (Abbas et al., 2013; Mazzella et al., 2014; Žádníková et al., 2015).
Ethylene acts directly upstream of auxin in the regulation of apical hook development, with HLS1 functioning as a key factor between both plant hormones (Lehman et al., 1996; Stowe-Evans et al., 1998; Li et al., 2004). Auxin is able to restore the defective hook formation in the ethylene-insensitive mutant ein2, indicating the importance of ethylene-mediated auxin biosynthesis in the regulation of hook development. In support, ethylene was shown to promote auxin production at the concave side of the hook through up-regulation of the auxin biosynthesis genes TRYPTOPHAN AMINOTRANSFERASE1 and TRYPTOPHAN AMINOTRANSFERASE-RELATED2 (Vandenbussche et al., 2010). Furthermore, the regulation of hook formation by ethylene depends on auxin transport. Inhibition of auxin influx as well as efflux hindered hook formation and exaggeration by ethylene. Moreover, ethylene affects apical hook development by regulating auxin transport. The expression of the auxin influx carriers AUXIN RESISTANT1 (AUX1)/LIKE AUXIN RESISTANCE3 and the auxin efflux carrier PIN-FORMED3 (PIN3) was enhanced, while that of PIN1 and PIN4 was reduced, by ethylene (Vandenbussche et al., 2010; Žádníková et al., 2010). BR, GA, and JA also impinge on auxin biosynthesis, transport, and signaling in the regulation of auxin distribution in the apical hook, whether or not through ethylene (De Grauwe et al., 2005; Gallego-Bartolomé et al., 2011; An et al., 2012a; Smet et al., 2014; Zhang et al., 2014b).
Upstream of auxin, GAs interact with ethylene in the regulation of apical hook development. Both hormones promote hook formation and prevent hook opening (Fig. 1; Vandenbussche et al., 2010; Žádníková et al., 2010; Gallego-Bartolomé et al., 2011; Smet et al., 2014). GA biosynthesis is indispensable for apical hook formation and the ethylene-induced apical hook exaggeration (Gallego-Bartolomé et al., 2011; An et al., 2012a), and ethylene promotes GA biosynthesis in the apical hook (Mazzella et al., 2014). Moreover, ethylene biosynthesis is also targeted by GAs during hook development. GAs enhanced the expression of ACS5/ETO2 and ACS8 in etiolated Arabidopsis seedlings (Gallego-Bartolomé et al., 2011). della quintuple mutants exhibited an exaggerated apical hook curvature, which suggested that GAs promote the ethylene-mediated hook formation through the promotion of DELLA protein degradation. Ethylene insensitivity inhibited hook exaggeration in the della quintuple mutant, supporting an ethylene-dependent regulation of apical hook formation by GAs. Also, HLS1 proved indispensable for the constitutive apical hook exaggeration in the della quintuple mutant (An et al., 2012a). Both ethylene and GAs promoted HLS1 expression (Gallego-Bartolomé et al., 2011; An et al., 2012a). EIN3/EIL1 emerged as DELLA-associated transcription factors, leading An et al. (2012a) to postulate a GA-nullified DELLA inhibition of EIN3/EIL1 as the mechanism for the ethylene-GA cross talk in the regulation of apical hook formation. However, GA partially restored apical hook curvature of the ethylene-insensitive mutant ein2, also indicating an ethylene-independent GA regulation of hook formation (Gallego-Bartolomé et al., 2011). Apart from apical hook formation, GAs and ethylene are also involved in hook opening. Both in the ethylene-insensitive mutant ein2 and in the GA-insensitive mutant gai-1, hook maintenance is inhibited while hook opening is advanced, suggesting that both plant hormones postpone apical hook opening (Vandenbussche et al., 2010; Gallego-Bartolomé et al., 2011; Smet et al., 2014). In support, apical hook opening is even prevented in the della quintuple mutant upon the administration of ACC (Gallego-Bartolomé et al., 2011).
Recently, a cross talk between BRs and ethylene in the regulation of apical hook development emerged. Etiolated BR-deficient mutants are hookless (Chory et al., 1991; Kauschmann et al., 1996; Li et al., 1996; Szekeres et al., 1996). Furthermore, the auxin response in the hook changes when the biosynthesis of BRs is affected (De Grauwe et al., 2005). Ethylene failed to exaggerate the hook curvature upon the inhibition of BR biosynthesis, suggesting that BRs are indispensable for the ethylene regulation of hook formation (Fig. 1). Nevertheless, in the absence of ethylene, the hook is not exaggerated by BRs. The reduced exaggeration of apical hook curvature by ethylene in the presence of BRs suggests, however, that BRs down-regulate the ethylene activity in apical hook formation (De Grauwe et al., 2005; Smet et al., 2014). Apart from hook formation, BRs and ethylene also interact in the regulation of hook maintenance and opening. BRs postponed the transition between hook maintenance and opening and, therefore, similar to ethylene and GAs, hamper apical hook opening (Fig. 1; Vandenbussche et al., 2010; Gallego-Bartolomé et al., 2011; Smet et al., 2014). However, ethylene postponed hook opening in the absence of BR biosynthesis, suggesting that, similar to hook formation, BRs also down-regulate the ethylene activity in apical hook opening (Smet et al., 2014).
In contrast with ethylene, BRs, and GAs, JAs recently emerged as negative regulators of hook formation (Fig. 1). The JA regulation of apical hook development is also mediated by ethylene. JAs inhibited the exaggeration of apical hook curvature induced by enhanced ethylene signaling in etiolated seedlings. However, upon JA insensitivity, apical hook development and ethylene-induced hook exaggeration remained unaffected, indicating that JAs are dispensable for apical hook development and the ethylene activity therein (Ellis and Turner, 2002; Turner et al., 2002). The JA insensitivity of hls1 seedlings again suggested a cross talk between ethylene and JAs at the level of HLS1. Consistently, JAs were shown to affect HLS1 expression in an EIN3/EIL1-dependent manner. MYC2 was subsequently shown to impede EIN3/EIL1 transcriptional activity through direct binding with the promoter of EIN3. Hence, JAs are assumed to counteract ethylene in the regulation of apical hook development by inhibiting the EIN3/EIL1-mediated HLS1 expression through the binding of MYC2 to EIN3 (Song et al., 2014; Zhang et al., 2014b).
ETHYLENE AND HYPOCOTYL GROWTH
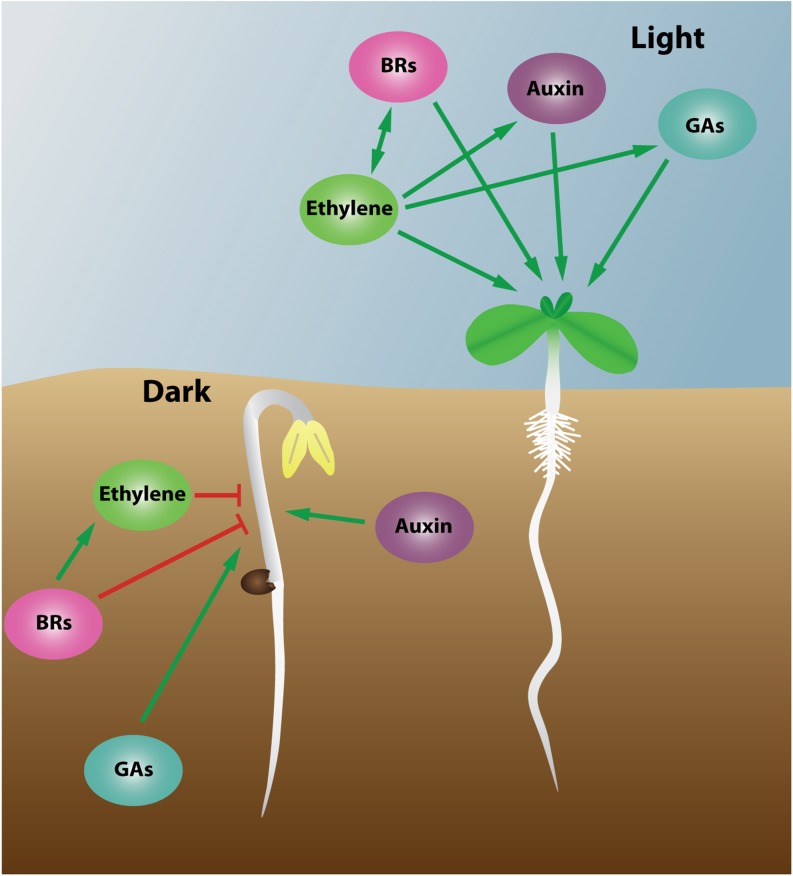
Ethylene regulates hypocotyl growth both in light and in darkness. In the dark, ethylene inhibits, whereas in the light, it promotes, the growth of Arabidopsis hypocotyls (Fig. 2). Light-grown eto2, ctr1, and EIN3-overexpressing seedlings had longer hypocotyls, whereas the hypocotyls of ethylene-insensitive etr1, ein2, ein3, and ein3 eil1 seedlings were shorter (Smalle et al., 1997; Alonso et al., 1999; Zhong et al., 2012; Yu et al., 2013). During skotomorphogenesis, hypocotyl growth is promoted, whereas during photomorphogenesis, it is inhibited, which is opposite to the effect of ethylene during the respective developmental programs. Consequently, a light-dependent regulation of hypocotyl growth by ethylene is expected.
Figure 2.
Simplified cross talk diagram showing the effects of and interactions between plant hormones in the regulation of hypocotyl development. The effect of the plant hormones on elongation and their interactions often differ between light and darkness. The hormonal cross talk is less well established in darkness compared with light. Ethylene plays an important role in the regulation of hypocotyl development, in the light and presumably also in darkness, interacting with other plant hormones. Red arrows indicate inhibitory effects, whereas green arrows indicate stimulatory effects.
In the dark, photomorphogenesis is repressed by CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1; Leivar and Quail, 2011; Lau and Deng, 2012). COP1 proved to function downstream of EIN3 in the light. Conversely, in darkness, COP1 acts upstream of EIN3. Both ethylene and overexpression of EIN3 reduced the expression of ETHYLENE RESPONSE FACTOR1 (ERF1) in etiolated wild-type seedlings, whereas in dark-grown cop1 seedlings, ERF1 expression was enhanced, suggesting that COP1 regulates the transcriptional activity of EIN3 (Liang et al., 2012).
Apart from COP1, photomorphogenesis is also repressed in the dark by PHYTOCROME INTERACTING FACTORS (PIFs; Leivar and Quail, 2011; Lau and Deng, 2012). PIF3 proved to be indispensable for the regulation of hypocotyl extension by ethylene in the light, with EIN3 promoting PIF3 expression by directly binding to its promoter. In darkness, the ethylene-induced inhibition of hypocotyl elongation is also mediated by EIN3/EIL1, although independently of PIF3. Both in light and in darkness, ethylene enhanced ERF1 expression. Nevertheless, overexpression of ERF1 substantially inhibited hypocotyl elongation in the dark, suggesting that ethylene depends on ERF1 for the inhibition of hypocotyl growth in the dark (Zhong et al., 2012).
As opposed to COP1 and PIFs, LONG HYPOCOTYL5 (HY5) transcription factors promote photomorphogenesis (Leivar and Quail, 2011; Lau and Deng, 2012), which suggests their involvement in the regulation of hypocotyl elongation. In support, ethylene was shown to depend on HY5 in the regulation of hypocotyl growth in the light but not in darkness (Liang et al., 2012; Yu et al., 2013). HY5 emerged as an inhibitor of ethylene during hypocotyl growth in the light. As for PIF3 (Zhong et al., 2012), HY5 appeared to act downstream of EIN3 in the regulation of hypocotyl growth by ethylene in the light. Moreover, ethylene suppressed the accumulation but not the expression of HY5. Ethylene promoted the nuclear localization of COP1 in the light, driving the degradation of HY5 (Yu et al., 2013). Hence, COP1, PIF3, and HY5 implement light and ethylene in the regulation of hypocotyl growth. Vandenbussche et al. (2007) demonstrated that the regulation of hypocotyl elongation by ethylene is blue light dependent and mediated by cryptochrome signaling.
Ethylene Cross Talk during Hypocotyl Growth
Hypocotyl growth is regulated by a complex hormone cross talk involving, apart from ethylene, also auxins, BRs, GAs, ABA, and CKs. Some of these plant hormones cooperate with ethylene therein. However, mutual interactions independent of ethylene were also demonstrated. As for ethylene, auxin is able to both inhibit and promote the elongation of Arabidopsis hypocotyls (Smalle et al., 1997; Collett et al., 2000). Inhibition of auxin efflux hinders hypocotyl elongation in the light, whereas in darkness, the effect is negligible (Garbers et al., 1996; Lehman et al., 1996; Jensen et al., 1998). However, hypocotyl elongation was significantly inhibited in dark-grown auxin-resistant mutants (Liang et al., 2012). Auxin biosynthesis, transport, and signaling proved to be important for ethylene-induced hypocotyl growth in the light (Fig. 2). Auxin accumulation was enhanced by ethylene, concomitant with an increased expression of the auxin biosynthesis genes YUCCA1 and YUCCA5. The inhibition of auxin efflux suppressed ethylene-induced hypocotyl elongation in the light. Ethylene also up-regulated the expression of the auxin transport genes AUX1, PIN3, and PIN7 in light-grown seedlings. Furthermore, the elongation of the hypocotyl in response to ethylene was inhibited in auxin-insensitive mutants in the light (Liang et al., 2012). Hence, the cross talk between ethylene and auxin in hypocotyl growth seems to be mediated by light, presumably via COP1 (Liang et al., 2012; Yu et al., 2013). Furthermore, HY5 has been shown to regulate the expression of genes involved in auxin signaling (Jing et al., 2013).
GAs stimulate hypocotyl elongation mainly by targeting the degradation of DELLA proteins, which negatively affect growth (Schwechheimer, 2008; Hauvermale et al., 2012). In light-grown Arabidopsis seedlings, GAs promote hypocotyl elongation (Fig. 2), whereas in etiolated seedlings, hypocotyl lengths remain unaltered. However, GA levels are significantly higher in etiolated seedlings, promoting hypocotyl elongation, which explains the absence of additional stimulation of hypocotyl elongation by exogenous GAs (Cowling and Harberd, 1999; Gendreau et al., 1999; Saibo et al., 2003). Consistently, upon inhibition of GA biosynthesis, GA significantly enhanced hypocotyl elongation in dark-grown Arabidopsis seedlings (Zhang et al., 2010; Fig. 2). The importance of GAs for hypocotyl elongation in the dark is also supported by the deetiolated growth of GA biosynthesis and signaling mutants in darkness and the wild type treated with the GA biosynthesis inhibitor paclobutrazol (Alabadí et al., 2004; Vriezen et al., 2004). As for ethylene, GAs negatively and positively regulate HY5 and PIFs, respectively, through COP1 or DELLAs (Jing et al., 2013; Zhang et al., 2014a). Hence, a cross talk between ethylene and GAs in the light-mediated regulation of hypocotyl elongation is expected. Consistently, in light-grown Arabidopsis seedlings, hypocotyl elongation is even more enhanced when ethylene and GA are administered together (Saibo et al., 2003). In blue light, the inhibition of GA biosynthesis completely abolished the ethylene-induced hypocotyl elongation, suggesting a GA-dependent regulation (Vandenbussche et al., 2007).
Like GAs, BRs are growth-promoting hormones. Arabidopsis BR biosynthesis and perception mutants have a dwarfed stature and are constitutively photomorphogenic in the dark (Chory et al., 1991; Kauschmann et al., 1996; Li et al., 1996; Szekeres et al., 1996). BRs promote hypocotyl elongation in the light, whereas in darkness, depending on the dose, hypocotyl lengths are unaffected or even reduced (Fig. 2). In the dark, the ethylene-induced inhibition of hypocotyl elongation was not promoted by BRs (Chen et al., 2013). Furthermore, ethylene insensitivity, but not deficiency, abolished the BR-induced inhibition of hypocotyl elongation (Deslauriers and Larsen, 2010). Hence, in the dark, an enhanced ethylene response mediates the BR-induced inhibition of hypocotyl elongation (Fig. 2). In the light, BRs further enhanced hypocotyl elongation induced by ethylene, and BR deficiency reduced the hypocotyl length in response to ethylene (Chen et al., 2013). Furthermore, the BR-induced hypocotyl elongation in the light was suppressed upon inhibition of ethylene perception. Ethylene and BRs thus appear to work antagonistically and interdependently in the regulation of hypocotyl elongation in the light (Fig. 2). The receptor-like kinase FERONIA was suggested to be a key modulator of the BR and ethylene responses in hypocotyl growth (Deslauriers and Larsen, 2010).
ETHYLENE AND PRIMARY ROOT GROWTH
Since the discovery of the triple response, it has become well established that ethylene plays an important role during primary root growth (Smalle and Van Der Straeten, 1997). More recent work with an octuple acs mutant revealed a slightly smaller root phenotype and an increased sensitivity toward externally applied ACC (Tsuchisaka et al., 2009), suggesting that a small amount of ethylene production is essential for normal root development.
Ethylene affects primary root growth at two different levels. First, ethylene induces stem cell division in the RAM (Ortega-Martínez et al., 2007). Second, ethylene inhibits cell expansion in the root elongation zone (Le et al., 2001). The action of ethylene on primary root growth is the net result of a complex cross talk with other hormones. Currently, the ethylene-auxin cross talk is best established, but more recent studies also highlight that ethylene interacts with other hormones during root development.
Ethylene Cross Talk during Primary Root Growth
The auxin-ethylene cross talk during root growth has been primarily characterized at the molecular level by Stepanova et al. (2007), Swarup et al. (2007), and Růzicka et al. (2007). Ultimately, this led to a hormone interaction model that has been reviewed by several authors (Benková and Hejátko, 2009; Vanstraelen and Benková, 2012; Takatsuka and Umeda, 2014). An important aspect of primary root development is the establishment of an auxin gradient along the longitudinal root axis. Polar transport drives the auxin flux through the inner stele from the hypocotyl toward the RAM in the QC, where a local auxin maximum drives the maintenance of the meristem and pattern formation. Subsequently, auxin is transported back upward, predominantly through the epidermis cells by means of auxin carriers, creating an auxin gradient and determining cell fate along the root axis. This auxin gradient is strictly regulated, and ethylene is one of the important players that regulates the auxin flow (Fig. 3). Over the years, an ethylene-auxin interaction model has been proposed for primary root growth. Ethylene can stimulate local auxin biosynthesis in the root apex and stimulate shootward auxin transport. Ethylene also enhances the auxin sensitivity of cells in the elongation zone. Altogether, ethylene is required for the establishment of a normal auxin gradient. Abnormal levels of ethylene will result in an imbalance in the auxin gradient, which in turn will lead to a higher auxin content in the elongation zone, resulting in the inhibition of cell elongation, a typical ethylene feature.
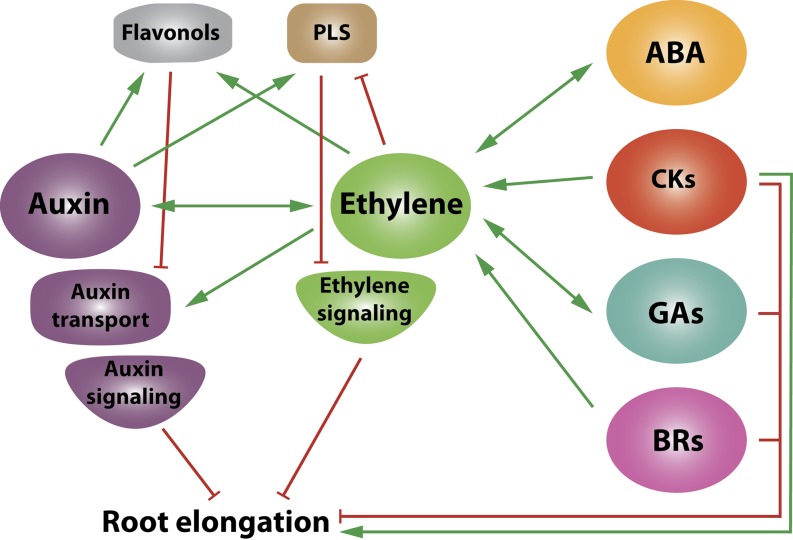
Figure 3.
Simplified cross talk diagram showing the interaction model between ethylene and other plant hormones that regulate primary root development through the modulation of cell elongation. Ethylene has a central hub position acting downstream of ABA, CKs, GAs, and BRs. Ethylene, in its turn, regulates primarily auxin biosynthesis, transport, and signaling, which is crucial for the establishment of the auxin gradient in the root, which drives the regulation of cell elongation. Two intermediate signaling molecules that affect root development and that are positioned between ethylene and auxins are flavonols and PLS. Red arrows indicate inhibitory effects, whereas green arrows indicate stimulatory effects.
It is important to note that two intermediate signaling components can intervene with the ethylene-auxin cross talk. The first group of secondary messengers is flavonols, which inhibit shootward auxin transport and cause the inhibition of root elongation (Muday et al., 2012; Fig. 3). Both ethylene and auxin can induce flavonol biosynthesis via two independent signaling pathways that converge through the MYB12 transcription factor (Lewis et al., 2011b). A second important messenger is POLARIS (PLS), a peptide acting as a negative regulator of the ethylene-induced inhibition of root growth (Casson et al., 2002; Chilley et al., 2006; Fig. 3). Ethylene down-regulates PLS expression, while auxins up-regulate PLS expression (Chilley et al., 2006). The pls mutant shows a short-root phenotype and increased levels of the auxin efflux carriers PIN1 and PIN2, while a PLS overexpression line shows a decrease in PIN1 and PIN2 levels (Liu et al., 2013). The double mutant pls etr1-1 did not show altered PIN levels, suggesting that PLS-mediated changes in PIN levels are regulated by ethylene signaling, which in turn alters the auxin gradient and, therefore, root growth (Liu et al., 2013).
GAs also play an important role in root growth. GAs promote the degradation of DELLA proteins, which were shown to accumulate in the endodermal cells of the elongation zone, where they inhibit root growth (Davière and Achard, 2013). Recently, it was shown that fluorescein-coupled GA also accumulates in the endodermal cells of the elongation zone, where it likely regulates DELLA stability. Interestingly, ACC treatment inhibited fluorescein-coupled GA accumulation, while having no effect on the fluorescein-coupled GA levels in ein2-5, suggesting that GA transport in the root epidermal cells is regulated by ethylene (Shani et al., 2013).
CKs also regulate root growth via cross talk with ethylene (Fig. 3). Originally, it was shown that CKs inhibit root growth and that this inhibition was mediated via the ethylene signaling pathway (Cary et al., 1995). Furthermore, CKs can induce ethylene biosynthesis by stabilizing ACS5 protein levels (Chae et al., 2003), resulting in a higher endogenous ACC content (Žd’árská et al., 2013). CKs also up-regulate the levels of S-adenosyl methionine synthetase and ACO in the root (Žd’árská et al., 2013), most likely leading to a local increase in ethylene production and, consequently, to the inhibition of root elongation. Contradictorily, Kushwah et al. (2011) showed that CKs can induce root elongation instead of inhibition and that this response is also mediated by the ethylene signaling pathway. More research will be needed to unravel these aspects of CK-ethylene cross talk on root development.
It was originally shown that ABA inhibits root growth and that this ABA response is mediated by the ethylene signaling pathway but not by ethylene biosynthesis (Beaudoin et al., 2000; Ghassemian et al., 2000; Thole et al., 2014). Recent reports have shown that ABA can stabilize the C-terminal part of ACS6, leading to higher ethylene production and, consequently, inhibition of root elongation (Luo et al., 2014). On the other hand, the ABA signaling protein PHOSPHATASE2C ABSCISIC ACID-INSENSITIVE2 can dephosphorylate ACS6, directing it for degradation, thus lowering ethylene production (Ludwików et al., 2014; Fig. 3). Besides the dual role of ABA on ethylene biosynthesis, it was shown that plants with a lower ethylene production capacity (aminoethoxyvinylglycine-treated plants and acs multiple knockout lines) all have decreased ABA sensitivity with relation to inhibition of root growth, while the ethylene-overproducing mutant eto shows increased ABA sensitivity (Luo et al., 2014). Thus, ABA can mediate ethylene biosynthesis, and it inhibits root elongation through the downstream action of ethylene.
BRs also play a dual role in root growth. Low concentrations of BRs can induce root growth, while high concentrations inhibit root growth, a response that is independent of ethylene (Clouse et al., 1996; Müssig et al., 2003). On the other hand, BRs can induce ethylene production in roots, suggesting that BRs can direct the ethylene-regulated inhibition of root growth (Müssig et al., 2003; Fig. 3). Recent work by Fridman et al. (2014) reported a cell type-specific cross talk interaction between ethylene and BRs. A cell type-specific expression of the BR receptor BRASSINOSTEROID INSENSITIVE1 (BRI1) in root hair cells induced cell elongation in all root cells, while a specific expression of BRI1 in nonhair cells inhibited root cell elongation. Moreover, nonhair cell expression of BRI1 appeared to induce ACS expression, and EIN2 was indispensable for the BRI1-induced root cell inhibition, suggesting that BR acts upstream of ethylene to inhibit root elongation.
Altogether, it is clear that ethylene interacts with other hormones to regulate primary root growth. A general interaction model where ethylene plays a central role is presented (Fig. 3). GAs, CKs, ABA, and BRs act (in part) upstream of ethylene, directing ethylene biosynthesis and/or requiring ethylene signaling in order to regulate the inhibition of root elongation, while auxin seems to act downstream of ethylene.
ETHYLENE AND LATERAL ROOT DEVELOPMENT
Besides primary root development, ethylene also plays an important role during the initiation and growth of lateral roots. It was initially shown that enhanced ethylene synthesis (upon ACC treatment or in eto1 mutants) or signaling (in ctr1 mutants) reduced lateral root formation. Ethylene-insensitive mutants (etr1 and ein2), on the other hand, enhance lateral root formation (Negi et al., 2008). Low doses of ACC promote the formation of lateral root primordia, as also evidenced by ACS1 expression (Rodrigues-Pousada et al., 1993), but the outgrowth of these primordia through the pericycle is prevented by ethylene (Ivanchenko et al., 2008). Similar to other vegetative developmental stages, ethylene does not act alone on lateral root development but interacts with other hormones.
Ethylene Cross Talk during Lateral Root Development
A major player in lateral root development is auxin, which promotes lateral root development. Auxin will prime pericycle cells, initiate cell cycle progression, stimulate asymmetric division, and ensure the emergence and elongation of the lateral root (for review, see Lavenus et al., 2013). Auxin and ethylene have antagonistic effects in lateral root development. Ethylene reduces DR5rev:GFP expression in the regions where lateral roots emerge, suggesting a locally reduced auxin responsiveness (Lewis et al., 2011a). Arabidopsis seedlings also show a local depletion of PIN3 and PIN7 abundance in the region just below developing lateral root primordia, matching the DR5rev:GFP expression pattern, suggesting a local depletion of auxin. This, in turn, results in a local auxin accumulation just above the lateral root primordia, giving rise to the formation of lateral roots (Lewis et al., 2011a; Aloni, 2013). Ethylene will prevent this local auxin depletion, predominantly by increasing PIN3 and PIN7 expression and abundance, thus increasing rootward auxin transport, which suppresses the local auxin maxima at lateral root primordia, inhibiting their outgrowth (Lewis et al., 2011a; Muday et al., 2012; Aloni, 2013).
ETHYLENE AND ADVENTITIOUS ROOT DEVELOPMENT
Adventitious roots are lateral roots initiated aboveground from the hypocotyl, instead of belowground from the primary root. In Arabidopsis, ethylene inhibits the formation of adventitious roots (Sukumar, 2010). Root-excised seedlings treated with ACC, as well as the eto1 and ctr1 mutants, showed fewer adventitious roots, while ethylene-insensitive mutants showed an increase in adventitious roots. On the other hand, ethylene inhibits auxin-stimulated adventitious root formation. It must be noted that, in other species, ethylene can stimulate adventitious root formation and that other hormones are also involved in the formation of adventitious roots (for review, see Bellini et al., 2014; Verstraeten et al., 2014).
ETHYLENE AND ROOT HAIR DEVELOPMENT
The development of root hairs is stimulated by ethylene (Tanimoto et al., 1995). Ethylene-insensitive etr1 and ein2 mutant alleles show shorter root hairs, while ethylene-overproducing eto1 mutants show longer root hairs, supporting that ethylene stimulates root hair elongation (Pitts et al., 1998; Rahman et al., 2002). Ethylene also regulates the differentiation of root epidermal cells, since the ctr1-1 mutant bears ectopic root hairs, as do wild-type roots treated with the ethylene precursor ACC (Tanimoto et al., 1995; Cao et al., 1999). The higher order acs mutants (hextuple and octuple mutants) exhibit an increased sensitivity toward ACC, reflected by an increase of root hair formation when treated with ACC (Tsuchisaka et al., 2009). The position of root hair emergence is also ethylene regulated, because the ethylene-overproducing mutant eto1 forms root hairs closer to the apical end of the root hair cell, while a dominant ethylene-insensitive etr1 mutant forms root hairs closer to the basal end of the root hair cell (Masucci and Schiefelbein, 1996). The exact location of root hair emergence is determined by the local auxin gradient, which, in turn, is partially regulated by ethylene (Ikeda et al., 2009). The action of ethylene (and other hormones like CKs) is at least in part mediated via the transcription factor C2H2 ZINC FINGER PROTEIN, which is a key regulator of root hair initiation and development in Arabidopsis (An et al., 2012b).
Auxin can also stimulate root hair formation independent from as well as in collaboration with ethylene (Grierson et al., 2014). For example, both auxin and ethylene can independently restore the root hair-deficient (rhd) phenotype of the rhd6 mutant (Masucci and Schiefelbein, 1994). On the other hand, auxin is able to rescue root hair defects in the ein2-1 mutant (Rahman et al., 2002). Auxin transport, and more precisely auxin influx via AUX1, is another point of cross talk that regulates root hair elongation, as evidenced by the suppression of the long-root-hair phenotype of the eto1 mutant in the eto1 aux1 double mutant (Strader et al., 2010).
Ethylene also interacts with JA to regulate root hair development (Zhu et al., 2006). JA biosynthesis inhibitors (ibuprofen and salicylhydroxamic acid) block the ACC-induced root hair formation, while ethylene inhibitors (Ag+ and aminoethoxyvinylglycine) block the JA-promoted formation of root hairs (Zhu et al., 2006).
CONCLUSION AND FUTURE PERSPECTIVES
Ethylene is a developmental regulator that operates in manifold physiological processes in all tissues throughout the plant life cycle. Compared with animals, plants have relatively few hormones, suggesting the necessity for hormonal cross talk. Over the years, hormonal biosynthesis and signaling pathways have been elucidated, allowing scientists to identify crucial players in hormonal interactions. This Update summarized key findings on ethylene and its cross talk with other hormones in the model species Arabidopsis, focusing on major developmental and growth phases of the vegetative life cycle and highlighting the different modes of action of ethylene in diverse tissues. Future challenges in the field of hormone research are grand, because it has become evident over the years that hormonal cross talk is not linear and should be tackled in a multidimensional space. This means that scientists should try to understand the three-dimensional spatial but also temporal relationships between two hormones, as well as the real-time concentration dependence, and their interdependent relationships with other hormones. The latter complex network of hormonal cross talk is not static but highly dynamic, again affected by time (development)- and space (cell and tissue specificity)-related factors. This Update highlighted the first studies where ethylene sensitivity and cross talk is cell type specific within a certain tissue and developmental stage. Future endeavors should provide more insight in these single-cell regulatory mechanisms.
Last but not least, the Gordian knot of hormonal cross talk dynamics is also affected by external clues such as, for example, light or temperature. Furthermore, it remains critical to precisely link hormone dynamics with a well-characterized phenotype. Major progress in the field of hormone biology will most likely be backed up by advancements in computer-aided phenotyping. The latter will enable plant scientists to further comprehend and visualize the vast hormonal cross talk dynamics.
Glossary
- QC
quiescent center
- RAM
root apical meristem
- CMT
cortical microtubules
- PCD
programmed cell death
- ACC
1-aminocyclopropane-1-carboxylic acid
- ROS
reactive oxygen species
- ABA
abscisic acid
- BR
brassinosteroid
- CK
cytokinin
- JA
jasmonate
Footnotes
This work was supported by the Research Foundation Flanders (grant nos. G.0306.12N and G.0656.13N to D.V.D.S.) and Ghent University. B.V.d.P. is a postdoctoral associate of Ghent University (Bijzonder Onderzoeksfonds). D.S. is a research assistant of the Research Foundation Flanders.
References
- Abbas M, Alabadí D, Blázquez MA (2013) Differential growth at the apical hook: all roads lead to auxin. Front Plant Sci 4: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadí D, Gil J, Blázquez MA, García-Martínez JL (2004) Gibberellins repress photomorphogenesis in darkness. Plant Physiol 134: 1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. (2013) Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta 238: 819–830 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- An F, Zhang X, Zhu Z, Ji Y, He W, Jiang Z, Li M, Guo H (2012a) Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res 22: 915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Zhou Z, Sun L, Yan A, Xi W, Yu N, Cai W, Chen X, Yu H, Schiefelbein J, et al. (2012b) A zinc finger protein gene ZFP5 integrates phytohormone signaling to control root hair development in Arabidopsis. Plant J 72: 474–490 [DOI] [PubMed] [Google Scholar]
- Andersson-Gunnerås S, Hellgren JM, Björklund S, Regan S, Moritz T, Sundberg B (2003) Asymmetric expression of a poplar ACC oxidase controls ethylene production during gravitational induction of tension wood. Plant J 34: 339–349 [DOI] [PubMed] [Google Scholar]
- Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A (2013) ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci 4: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashline L, Lei L, Li S, Gu Y (2014) Cell wall, cytoskeleton, and cell expansion in higher plants. Mol Plant 7: 586–600 [DOI] [PubMed] [Google Scholar]
- Baskin TI. (2015) Auxin inhibits expansion rate independently of cortical microtubules. Trends Plant Sci 20: 471–472 [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65: 639–666 [DOI] [PubMed] [Google Scholar]
- Benková E, Hejátko J (2009) Hormone interactions at the root apical meristem. Plant Mol Biol 69: 383–396 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle M, Sommerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 240: 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bollhöner B, Prestele J, Tuominen H (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63: 1081–1094 [DOI] [PubMed] [Google Scholar]
- Cao XF, Linstead P, Berger F, Kieber J, Dolan L (1999) Differential ethylene sensitivity of epidermal cells is involved in the establishment of cell pattern in the Arabidopsis root. Physiol Plant 106: 311–317 [DOI] [PubMed] [Google Scholar]
- Cary AJ, Liu W, Howell SH (1995) Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol 107: 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson SA, Chilley PM, Topping JF, Evans IM, Souter MA, Lindsey K (2002) The POLARIS gene of Arabidopsis encodes a predicted peptide required for correct root growth and leaf vascular patterning. Plant Cell 14: 1705–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ (2003) The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IJ, Lo WS, Chuang JY, Cheuh CM, Fan YS, Lin LC, Wu SJ, Wang LC (2013) A chemical genetics approach reveals a role of brassinolide and cellulose synthase in hypocotyl elongation of etiolated Arabidopsis seedlings. Plant Sci 209: 46–57 [DOI] [PubMed] [Google Scholar]
- Chen X, Grandont L, Li H, Hauschild R, Paque S, Abuzeineh A, Rakusová H, Benkova E, Perrot-Rechenmann C, Friml J (2014) Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 516: 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Chiang MH, Hwang SG, Lin PC (2009) Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol Biol 71: 61–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilley PM, Casson SA, Tarkowski P, Hawkins N, Wang KL, Hussey PJ, Beale M, Ecker JR, Sandberg GK, Lindsey K (2006) The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 18: 3058–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, Kermode AR (2005) The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42: 35–48 [DOI] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ (2002) Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 14: 3237–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Nagpal P, Peto CA (1991) Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3: 445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC (1996) A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111: 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett CE, Harberd NP, Leyser O (2000) Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol 124: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H (2014) Ethylene, a key factor in the regulation of seed dormancy. Front Plant Sci 5: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2000) Loosening of plant cell walls by expansins. Nature 407: 321–326 [DOI] [PubMed] [Google Scholar]
- Cowling RJ, Harberd NP (1999) Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J Exp Bot 50: 1351–1357 [Google Scholar]
- Darwin C, Darwin F (1881). The Power of Movement in Plants. D Appleton, New York [Google Scholar]
- Davière JM, Achard P (2013) Gibberellin signaling in plants. Development 140: 1147–1151 [DOI] [PubMed] [Google Scholar]
- De Grauwe L, Vandenbussche F, Tietz O, Palme K, Van Der Straeten D (2005) Auxin, ethylene and brassinosteroids: tripartite control of growth in the Arabidopsis hypocotyl. Plant Cell Physiol 46: 827–836 [DOI] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB (2010) FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant 3: 626–640 [DOI] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5: 123–127 [DOI] [PubMed] [Google Scholar]
- Ellis C, Turner JG (2002) A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta 215: 549–556 [DOI] [PubMed] [Google Scholar]
- El-Maarouf-Bouteau H, Sajjad Y, Bazin J, Langlade N, Cristescu SM, Balzergue S, Baudouin E, Bailly C (2014) Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ 38: 364–374 [DOI] [PubMed] [Google Scholar]
- Etchells JP, Provost CM, Turner SR (2012) Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. PLoS Genet 8: e1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman Y, Elkouby L, Holland N, Vragović K, Elbaum R, Savaldi-Goldstein S (2014) Root growth is modulated by differential hormonal sensitivity in neighboring cells. Genes Dev 28: 912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Gallego-Bartolomé J, Arana MV, Vandenbussche F, Zádníková P, Minguet EG, Guardiola V, Van Der Straeten D, Benková E, Alabadí D, Blázquez MA (2011) Hierarchy of hormone action controlling apical hook development in Arabidopsis. Plant J 67: 622–634 [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruére J, Bernasconi P, Söll D (1996) A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J 15: 2115–2124 [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Orbovic V, Höfte H, Traas J (1999) Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta 209: 513–516 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson C, Nielsen E, Ketelaarc T, Schiefelbein J (2014) Root hairs. The Arabidopsis Book 12: e0172, doi/10.1199/tab.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, Grebe M (2009) Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol 11: 731–738 [DOI] [PubMed] [Google Scholar]
- Hauvermale AL, Ariizumi T, Steber CM (2012) Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiol 160: 83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55: 335–347 [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M (1998) Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Zhang D, Wang X, Tang W, Wang W, Huai J, Xu G, Chen D, Li Y, Lin R (2013) Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25: 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T (1996) Genetic evidence for an essential role of brassinosteroids in plant development. Plant J 9: 701–713 [Google Scholar]
- Kushwah S, Jones AM, Laxmi A (2011) Cytokinin interplay with ethylene, auxin, and glucose signaling controls Arabidopsis seedling root directional growth. Plant Physiol 156: 1851–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17: 584–593 [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18: 450–458 [DOI] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, De Cnodder T, Van Der Straeten D, Verbelen JP (2005) Cell elongation and microtubule behavior in the Arabidopsis hypocotyl: responses to ethylene and auxin. J Plant Growth Regul 24: 166–178 [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP (2001) In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol 125: 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP (2004) Position and cell type-dependent microtubule reorientation characterizes the early response of the Arabidopsis root epidermis to ethylene. Physiol Plant 121: 513–519 [Google Scholar]
- Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85: 183–194 [DOI] [PubMed] [Google Scholar]
- Leivar P, Quail PH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK (2011a) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Ramirez MV, Miller ND, Vallabhaneni P, Ray WK, Helm RF, Winkel BSJ, Muday GK (2011b) Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol 156: 144–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leymarie J, Vitkauskaité G, Hoang HH, Gendreau E, Chazoule V, Meimoun P, Corbineau F, El-Maarouf-Bouteau H, Bailly C (2012) Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol 53: 96–106 [DOI] [PubMed] [Google Scholar]
- Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR (2004) Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell 7: 193–204 [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401 [DOI] [PubMed] [Google Scholar]
- Liang X, Wang H, Mao L, Hu Y, Dong T, Zhang Y, Wang X, Bi Y (2012) Involvement of COP1 in ethylene- and light-regulated hypocotyl elongation. Planta 236: 1791–1802 [DOI] [PubMed] [Google Scholar]
- Linkies A, Leubner-Metzger G (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31: 253–270 [DOI] [PubMed] [Google Scholar]
- Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, et al. (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21: 3803–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Mehdi S, Topping J, Friml J, Lindsey K (2013) Interaction of PLS and PIN and hormonal crosstalk in Arabidopsis root development. Front Plant Sci 4: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ye N, Liu R, Chen M, Zhang J (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61: 2979–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J, Björklund S, Vahala J, Hertzberg M, Kangasjärvi J, Sundberg B (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci USA 106: 5984–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwików A, Cieśla A, Kasprowicz-Maluśki A, Mituła F, Tajdel M, Gałgański Ł, Ziółkowski PA, Kubiak P, Małecka A, Piechalak A, et al. (2014) Arabidopsis protein phosphatase 2C ABI1 interacts with type I ACC synthases and is involved in the regulation of ozone-induced ethylene biosynthesis. Mol Plant 7: 960–976 [DOI] [PubMed] [Google Scholar]
- Luo X, Chen Z, Gao J, Gong Z (2014) Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J 79: 44–55 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106: 1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci JD, Schiefelbein JW (1996) Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell 8: 1505–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzella MA, Casal JJ, Muschietti JP, Fox AR (2014) Hormonal networks involved in apical hook development in darkness and their response to light. Front Plant Sci 5: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Cox MCH, van Berkel YE, Welschen RAM, Pierik R, Voesenek LAJC, Peeters AJM (2005) Ethylene-induced differential growth of petioles in Arabidopsis: analyzing natural variation, response kinetics, and regulation. Plant Physiol 137: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miransari M, Smith DL (2014) Plant hormones and seed germination. Environ Exp Bot 99: 110–121 [Google Scholar]
- Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195 [DOI] [PubMed] [Google Scholar]
- Mühlenbock P, Plaszczyca M, Plaszczyca M, Mellerowicz E, Karpinski S (2007) Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 19: 3819–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müssig C, Shin GH, Altmann T (2003) Brassinosteroids promote root growth in Arabidopsis. Plant Physiol 133: 1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Martínez O, Pernas M, Carol RJ, Dolan L (2007) Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science 317: 507–510 [DOI] [PubMed] [Google Scholar]
- Pesquet E, Tuominen H (2011) Ethylene stimulates tracheary element differentiation in Zinnia elegans cell cultures. New Phytol 190: 138–149 [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16: 553–560 [DOI] [PubMed] [Google Scholar]
- Polko JK, van Rooij JA, Vanneste S, Pierik R, Ammerlaan AMH, Vergeer-van Eijk MH, McLoughlin F, Gühl K, Van Isterdael G, Voesenek LACJ, et al. (2015) Ethylene-mediated regulation of A2-type CYCLINs modulates hyponastic growth in Arabidopsis. Plant Physiol 169: 194–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polko JK, van Zanten M, van Rooij JA, Marée AF, Voesenek LA, Peeters AJ, Pierik R (2012) Ethylene-induced differential petiole growth in Arabidopsis thaliana involves local microtubule reorientation and cell expansion. New Phytol 193: 339–348 [DOI] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130: 1908–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos P, Herrera R (2013) Anatomical changes of xylem cells in stem of Pinus radiata seedlings exposed to inclination and ethylene. Biol Plant 57: 525–530 [Google Scholar]
- Rauf M, Arif M, Fisahn J, Xue GP, Balazadeh S, Mueller-Roeber B (2013) NAC transcription factor speedy hyponastic growth regulates flooding-induced leaf movement in Arabidopsis. Plant Cell 25: 4941–4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Ecker JR (1999) Regulation of differential growth in the apical hook of Arabidopsis. Development 126: 3661–3668 [DOI] [PubMed] [Google Scholar]
- Raz V, Koornneef M (2001) Cell division activity during apical hook development. Plant Physiol 125: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Pousada RA, De Rycke R, Dedonder A, Van Caeneghem W, Engler G, Van Montagu M, Van Der Straeten D (1993) The Arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell 5: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibo NJM, Vriezen WH, Beemster GTS, Van Der Straeten D (2003) Growth and stomata development of Arabidopsis hypocotyls are controlled by gibberellins and modulated by ethylene and auxins. Plant J 33: 989–1000 [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Voesenek LA, Pierik R (2011) Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. CRC Crit Rev Plant Sci 30: 548–562 [Google Scholar]
- Schwechheimer C. (2008) Understanding gibberellic acid signaling: are we there yet? Curr Opin Plant Biol 11: 9–15 [DOI] [PubMed] [Google Scholar]
- Shani E, Weinstain R, Zhang Y, Castillejo C, Kaiserli E, Chory J, Tsien RY, Estelle M (2013) Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc Natl Acad Sci USA 110: 4834–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Eloy NB, Coppens F, Yoo SD, et al. (2011) Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23: 1876–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D (1997) Ethylene and vegetative development. Physiol Plant 100: 593–605 [Google Scholar]
- Smet D, Žádníková P, Vandenbussche F, Benková E, Van Der Straeten D (2014) Dynamic infrared imaging analysis of apical hook development in Arabidopsis: the case of brassinosteroids. New Phytol 202: 1398–1411 [DOI] [PubMed] [Google Scholar]
- Song SS, Huang H, Gao H, Wang JJ, Wu DW, Liu XL, Yang SH, Zhai QZ, Li CY, Qi TC, et al. (2014) Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26: 263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E (1998) NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol 118: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader LC, Chen GL, Bartel B (2010) Ethylene directs auxin to control root cell expansion. Plant J 64: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IH, Aman S, Zubo Y, Ramzan A, Wang X, Shakeel SN, Kieber JJ, Schaller GE (2015) Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol 169: 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V, Reddy KJ (2010) Interactions between ethylene, abscisic acid and cytokinin during germination and seedling establishment in Arabidopsis. J Biosci 35: 451–458 [DOI] [PubMed] [Google Scholar]
- Sukumar P (2010) The role of auxin and ethylene in adventitious root formation in Arabidopsis and tomato. PhD thesis. Wake Forest University, Winston-Salem, NC [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182 [DOI] [PubMed] [Google Scholar]
- Takatsuka H, Umeda M (2014) Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot 65: 2633–2643 [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8: 943–948 [DOI] [PubMed] [Google Scholar]
- Thole JM, Beisner ER, Liu J, Venkova SV, Strader LC (2014) Abscisic acid regulates root elongation through the activities of auxin and ethylene in Arabidopsis thaliana. G3 Genes Genomes Genet 4: 1259–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann A, Lechner E, Hansen M, Dumbliauskas E, Parmentier Y, Kieber J, Scheres B, Genschik P (2009) Arabidopsis CULLIN3 genes regulate primary root growth and patterning by ethylene-dependent and -independent mechanisms. PLoS Genet 5: e1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, Gao S, Theologis A (2009) A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183: 979–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell (Suppl) 14: S153–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Petrásek J, Zádníková P, Hoyerová K, Pesek B, Raz V, Swarup R, Bennett M, Zazímalová E, Benková E, et al. (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development 137: 597–606 [DOI] [PubMed] [Google Scholar]
- Vandenbussche F, Vancompernolle B, Rieu I, Ahmad M, Phillips A, Moritz T, Hedden P, Van Der Straeten D (2007) Ethylene-induced Arabidopsis hypocotyl elongation is dependent on but not mediated by gibberellins. J Exp Bot 58: 4269–4281 [DOI] [PubMed] [Google Scholar]
- Van Hautegem T, Waters AJ, Goodrich J, Nowack MK (2015) Only in dying, life: programmed cell death during plant development. Trends Plant Sci 20: 102–113 [DOI] [PubMed] [Google Scholar]
- Vanstraelen M, Benková E (2012) Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol 28: 463–487 [DOI] [PubMed] [Google Scholar]
- Verstraeten I, Schotte S, Geelen D (2014) Hypocotyl adventitious root organogenesis differs from lateral root development. Front Plant Sci 5: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Fry SC, Verbelen JP (2001) Root hair initiation is coupled to a highly localized increase of xyloglucan endotransglycosylase action in Arabidopsis roots. Plant Physiol 127: 1125–1135 [PMC free article] [PubMed] [Google Scholar]
- Vriezen W, Achard P, Harberd N, Van Der Straeten D (2004) Ethylene-mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. Plant J 37: 505–516 [DOI] [PubMed] [Google Scholar]
- Wilson RL, Bakshi A, Binder BM (2014a) Loss of the ETR1 ethylene receptor reduces the inhibitory effect of far-red light and darkness on seed germination of Arabidopsis thaliana. Front Plant Sci 5: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RL, Kim H, Bakshi A, Binder BM (2014b) The ethylene receptors ETHYLENE RESPONSE1 and ETHYLENE RESPONSE2 have contrasting roles in seed germination of Arabidopsis during salt stress. Plant Physiol 165: 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35: 155–189 [Google Scholar]
- Yu Y, Wang J, Zhang Z, Quan R, Zhang H, Deng XW, Ma L, Huang R (2013) Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genet 9: e1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zádníková P, Petrásek J, Marhavy P, Raz V, Vandenbussche F, Ding Z, Schwarzerová K, Morita MT, Tasaka M, Hejátko J, et al. (2010) Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. Development 137: 607–617 [DOI] [PubMed] [Google Scholar]
- Žádníková P, Smet D, Zhu Q, Van Der Straeten D, Benková E (2015) Strategies of seedlings to overcome their sessile nature: auxin in mobility control. Front Plant Sci 6: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žd’árská M, Zatloukalová P, Benítez M, Šedo O, Potěšil D, Novák O, Svačinová J, Pešek B, Malbeck J, Vašíčková J, et al. (2013) Proteome analysis in Arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis. Plant Physiol 161: 918–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jing Y, Jiang Z, Lin R (2014a) The chromatin-remodeling factor PICKLE integrates brassinosteroid and gibberellin signaling during skotomorphogenic growth in Arabidopsis. Plant Cell 26: 2472–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhu Z, An F, Hao D, Li P, Song J, Yi C, Guo H (2014b) Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 26: 1105–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Z, Wang L, Zheng S, Xie J, Bi Y (2010) Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J Plant Physiol 167: 1130–1136 [DOI] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wang L, Xi Y, Li J, Quail PH, Deng XW, Guo H (2012) A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol 22: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Gan L, Shen Z, Xia K (2006) Interactions between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J Exp Bot 57: 1299–1308 [DOI] [PubMed] [Google Scholar]