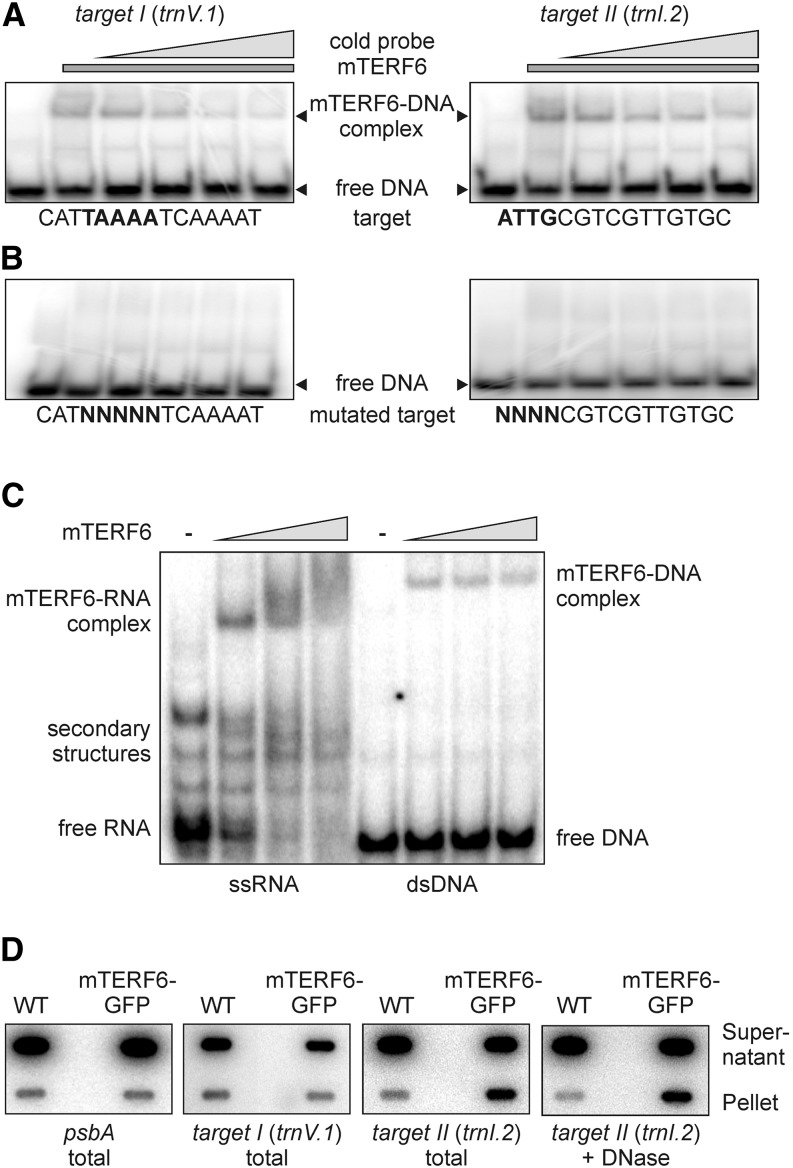
Figure 6.
The mTERF6 protein interacts with a nucleic acid sequence located in the plastid trnI.2 gene (target II). A, EMSAs were performed with recombinant His6-tagged mTERF6. Aliquots (1 µg) of recombinant protein were incubated with 100 ng of [γ-32P]ATP-labeled dsDNA probes representing the target I and target II sequences of mTERF6 in the presence of increasing concentrations (up to 10-fold relative to the labeled probe; indicated by the gray triangles) of the same unlabeled probe as competitor. Binding reactions were then subjected to electrophoresis on nondenaturing TBE-polyacrylamide gels (see “Materials and Methods”). EMSAs for the other four putative target sequences are shown in Supplemental Figure S4. B, Demonstration of specific sequence binding. EMSA reactions were performed as in A with probes in which 4 or 5 bp within the target I or target II sequence had been replaced by random nucleotides (mutated target). C, EMSA showing that mTERF6 binds ssRNA matching the trnI.2/3 target (target II). Increasing concentrations (50, 250, and 500 nm; indicated by the gray triangles) of recombinant His6-tagged mTERF6 protein were incubated with 50 pm [γ-32P]ATP-labeled ssRNA and dsDNA probes corresponding to the target II sequence in the plastid trnI.2 gene. Binding reactions were fractionated on nondenaturing TBE-polyacrylamide gels. D, Coimmunoprecipitation analysis to investigate the in vivo binding properties of mTERF6 to target I and target II sequences. The detergent-treated stromal extracts of complemented mterf6-1 (35S:MTERF6.1 mterf6-1) and wild-type (WT; Col-0) plants were subjected to immunoprecipitation with GFP antibodies. Nucleic acids from the supernatant and the immunoprecipitation pellets were recovered (total) and for probing with target II in addition DNase treated (+ DNase). Nucleic acids were slot blotted onto a nylon membrane and probed with [α-32P]dCTP-labeled cDNA fragments specific for target I, target II, and psbA.