Figure 4. Motifs that define the αC-Helix.
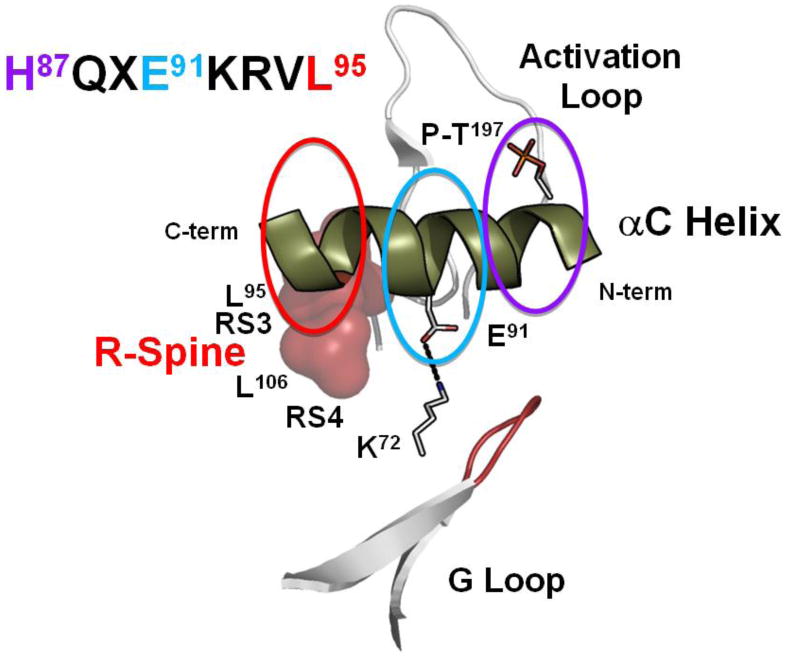
The extended αC Helix allows one to appreciate how essential residues are spread across the entire helix. While every residue is functionally important three specific features are highlighted here. The sequence of the αC Helix is shown on the top left with three key residues highlighted in red, teal and purple. RS3/L95 (red) lies at the C-terminus, the conserved salt bridge to Lys72, Glu91 (teal), is in the middle, and His87 (purple) at the N-terminus is anchored to the activation loop phosphate, (P)-Thr197.
