Abstract
Autophagy attenuates the efficacy of conventional chemotherapy but its effects on immunotherapy have been little studied. Here, we report that chemotherapy renders tumor cells more susceptible to lysis by CTL in vivo. Moreover, bystander tumor cells that did not express antigen were killed by CTL. This effect was mediated by transient but dramatic upregulation of the mannose-6-phosphate receptor (MPR) on the tumor cell surface. Antitumor effects of combined treatment related to the kinetics of MPR upregulation and abrogation of this event abolished the combined effect of immunotherapy and chemotherapy. MPR accumulation on the tumor cell surface during chemotherapy was observed in different mouse tumor models and in patients with multiple myeloma. Notably, this effect was the result of redistribution of the receptor caused by chemotherapy-inducible autophagy. Together, our findings reveal one molecular mechanism through which the antitumor effects of conventional cancer chemotherapy and immunotherapy are realized.
Introduction
Therapeutic cancer vaccines and adoptive T-cell transfer are attractive options for the treatment of different types of cancer. In recent years, it has become apparent that cancer immune therapy may provide only limited clinical benefits and that its combination with targeted or cytotoxic chemotherapeutic treatment will be necessary to achieve substantial clinical benefit. However, the use of conventional cancer chemotherapy in combination with immunotherapy was previously not deemed appropriate because of potent immunosuppressive effects usually associated with chemotherapy. This paradigm was challenged in recent years by the serendipitous observations made in a number of phase I/II clinical trials in which high rates of objective clinical responses were observed when cancer vaccines were combined with chemotherapy (1–5). These observations were made using various cancer vaccines and different chemotherapeutic regimens in patients with diverse types of cancer (6). The mechanisms of the potential beneficial effect of combined immunochemotherapy remain unknown.
In animal tumor models, it has been shown that conventional chemotherapy and radiation therapy can induce immune responses against antigens generated in dying tumor cells (7, 8). Single injections of chemotherapeutic drugs may induce an antitumor immune response by directly causing immunogenic cell death (9). When used in noncytotoxic doses, drugs such as paclitaxel, doxorubicin, mitomycin C, and methotrexate increased antigen presentation by antigen presenting cells (10). Dendritic cells (DC), treated with vinblastine, underwent maturation and exhibited better ability to induce CD8 T-cell responses compared with untreated DCs (11). Chemotherapy has also been shown to render cancer cells more susceptible to killing by CTLs. 5-fluorouracil, CPT-11, and cisplatin were all shown to increase the sensitivity of the SW480 colon cancer cell line to killing by T cells (12). Cytotoxic drugs can modulate systemic immune suppression or expand antigen-specific T-cells via cytoreduction (reviewed in ref. 13). However, the paradox is that the chemotherapeutic agents used in those studies are known to suppress immunity in cancer patients during treatment, which includes multiple repeated doses of drugs (14). There is ample evidence that chemotherapeutic agents can ablate T-cell function and blunt antitumor immune responses (15). Previous studies indicated that conventional doses of chemotherapy did not support the development and maintenance of antitumor immune responses (16). Even in patients who benefited from combined treatment, chemotherapy inhibited antigen-specific T cells generated by previously administered cancer vaccines (2). Moreover, combination of chemotherapy with high-dose cytokines has failed to improve survival of patients with metastatic melanoma (17).
These observations suggested that chemotherapy can potentiate immunity if used in either single dose or in low noncytotoxic doses. However, the critical question is: Can immunotherapy be used in combination with conventional chemotherapy in patients with advanced stage cancer?
We recently showed that chemotherapy makes tumor cells permeable to granzyme B (GrzB) released by activated CTLs in a perforin independent manner (18). We suggested that this effect in vitro could be mediated via upregulation of the cation-independent mannose-6-phosphate (MPR) receptor on the surface of tumor cells. MPR (also termed insulin-like growth factor 2 receptor) is a multifunctional protein with high-affinity binding to insulin-like growth factor 2. Within the cell, MPRs are found in the trans-Golgi network (TGN), endosomes, and are also present within the plasma membrane. On the membrane, these receptors bind to their ligands and the whole complex is packaged in transport intermediates consisting of clathrin-coated vesicles and the AP-1 assembly complex. Clathrin-coated vesicles mediate the sorting of MPRs from TGN for transportation to endosomal compartments. Within early endosomes, ligand dissociates from the receptor because of the low pH (19). The receptors are recycled back to TGN by the complex containing Golgi-localized, γ-ear containing ADP-ribosylation factor binding proteins (20). MPR function is associated with endocytosis and degradation (21). MPR was previously implicated in granzyme B delivery to cells, bypassing perforin (22). Although under normal conditions, MPR play a minor role in CTL-mediated killing (23–25), this receptor is considered to be an important factor that, together with perforin, mediates granzyme B entry into the cell (24, 26).
In this study, we investigated the role of MPR during combined chemotherapy and immune therapy of cancer in vivo.
Materials and Methods
Mice and tumor models
Animal protocols were approved by the University of South Florida Animal Care and Use Committee. Female C57BL/6J (B6, H-2b), BALB/c (H-2d), and Pmel mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA). Murine lymphoblastoma cell line, EL4 and B16-F10 melanoma, mammary carcinoma 4T1 lines were purchased from American Type Culture Collection (ATCC) after 2009. B16-F10Kb cells were isolated as an escape mutant from vaccinated mice (27). Small cell lung cancer cell line 86M1 and human multiple myeloma cell lines H929, U266, and 8226 were purchased from ATCC after 2008. The cells were maintained in RPMI 1640 media containing 10% FBS. B16-F10-luc-G5 mouse melanoma cell line that expresses the firefly luciferase was obtained from Xenogen and maintained with 200 μg/mL Zeocin (Invitrogen). Tumor cells were treated with 12.5 nmol/L paclitaxel, 25 ng/mL doxorubicin, and 25 ng/mL cisplatin for 18 hours before being used as targets in various assays.
Patients’ samples
Patients with a histologically confirmed diagnosis of multiple myeloma were enrolled during 2002–2006 to clinical protocol MCC12733 approved by the University of South Florida institutional review board. Patients were treated for 3 days with melphalan (50 mg/m2/d i.v.) and escalating doses of topotecan (starting at 10 mg/m2/d i.v.). Bone marrow samples were collected before and immediately after 3 days of high-dose chemotherapy. Cell suspensions from bone marrow aspirates were cytospun on slides and kept frozen in liquid nitrogen. Paired samples from 10 patients treated under this protocol were available for the analysis in our study.
Immunohistochemistry for MPR
Protocol is provided in Supplemental Materials.
Transfection of cell lines with MPR shRNA, ATG5siRNA, or ATG5shRNA vector
B16F10 and B16F10 kb cell lines were stably transfected with either a control plasmid short hairpin RNA (shRNA) or MPR or Atg5 shRNA vector incorporating the puromycin resistance gene for selection (Mission, Sigma-Aldrich) using Geneporter 2 kit (Genlantis). B16F10 cells were transfected with ATG5siRNA using the Nucleofector Kit C (Lonza; Program X-05 on Amaxa) and 300 nmol/L of atg5 siRNAs or with 300 nmol/L of scrambled siRNA. The cells were resuspended in Dulbecco’s Modified Eagle’s Media medium and rested for 48 hours before treatment with paclitaxel.
Detection of MPR and granzyme B in cell lines
Cell lines were treated with 12.5 nmol/L paclitaxel for 16 hours and labeled for MPR and granzyme B as described earlier (18). A portion of the cells was fixed with 2% paraformaldehye for 20 minutes at room temperature before staining. Dead cells were discriminated from the live population by either 4′, 6-diamidino-2-phenylindole stain or Live/Dead Fixable Dead cell stain kit (Invitrogen).
Treatment protocol for B16F10 control shRNA/MPR shRNA tumor models
C57BL/6 mice were inoculated with 0.5 × 106 tumor cells/mouse on day 0. Spleen cells from Pmel mice were activated with 0.1 μg/mL of gp-100 peptide in vitro for 72 hours and T cells were purified using nylon wool. On day 11, mice were injected intravenously with 5 × 106 of Pmel T cells. On day 14, paclitaxel was administered intraperitoneally at a dose of 12.5 mg/kg.
Luciferase murine model for bystander assay
Detailed protocol is provided in Supplemental Materials. For adoptive transfer using endogenous, vaccine-generated T cells, B16 tumor–bearing mice received 5 × 106 Trp2180-specific CD8+ T cells generated in B6 mice immunized with Trp2180 TriVax (27).
Quantitative real-time PCR and Western blotting
Protocol is provided in Supplementary Methods.
Confocal imaging
U266 multiple myeloma cells were treated with 25 ng/mL doxorubicin for 18 hours, cytospun and fixed with 4% paraformaldehyde for 30 minutes in humid chamber. The cells were blocked with 10% human serum for 30 minutes and then labeled with primary MPR antibody, followed by goat anti-rabbit Alexa 555 (Invitrogen). For inhibition of autophagy, the cells were treated with 1 mmol/L 3 methyladenine (3MA) for 4 hours before treatment with doxorubicin overnight and then stained as described above. The cells were imaged with a Leica TCS SP5 laser scanning confocal microscope through a 63X/1.40NA Plan Apochromat oil immersion objective lens (Leica Microsystems). Diode lasers lines of 405 and 555 nm were applied to excite the samples. An acousto-optical beam splitter was used to collect peak emission photons sequentially to minimize cross-talk between fluorochromes. Protocol is provided in Supplementary Methods.
Statistical analysis
Statistical analysis was conducted using a 2-tailed Student t test and GraphPad Prism 5 software (GraphPad Software Inc.), with significance determined at P < 0.05. Analysis of tumor growth curves was conducted, using a 2-way ANOVA test with a Bonferroni posttest.
Results
The antitumor effect of combined chemoimmunotherapy is linked to a transient induction of MPR
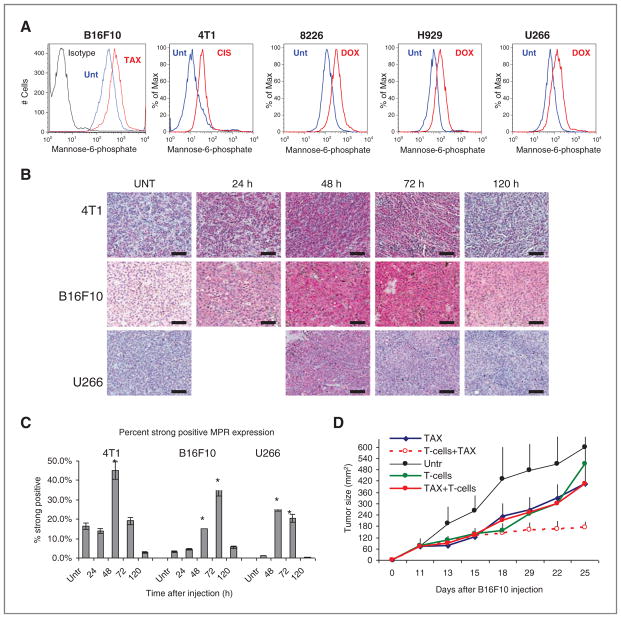
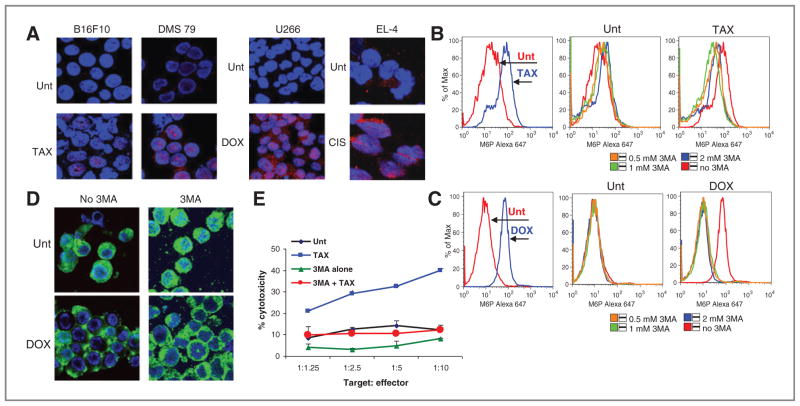
We tested the effects of chemotherapy on the expression of MPR in vivo using several mouse (B16F10 melanoma, 4T1 mammary carcinoma) and human (multiple myeloma 8226, H929, and U266) tumor cell lines. Consistent with the results of our previous study (18), different chemotherapeutic agents such as paclitaxel, doxorubicin, and cisplatin caused substantial, and similar upregulation of MPR expression in vitro in all tested mouse and human tumor cell lines (Fig. 1A).
Figure 1.
Chemotherapy causes transient upregulation of MPR in tumors. A, upregulation of MPR in vitro on the surface of indicated tumor cells after overnight treatment with 12.5 nmol/L paclitaxel (TAX; B16F10 and 4T1) or 25 ng/mL doxorubicin (DOX; 8226, H929, and U266). Typical examples of 3 to 5 conducted experiments are shown. Staining with isotype IgG showed less than 101 fluorescence intensity (not shown). B, effect of chemotherapy on MPR expression in tumors in vivo. 4T1 and B16F10 tumor-bearing mice were treated with 12.5 mg/kg paclitaxel and U266 tumor-bearing NOD/ SCID mice were treated with 25 mg/kg doxorubicin. Tumors were excised at indicated times after chemotherapy and stained for MPR. Bar, 100 μm. C, mean ± SD of cumulative results are shown. Quantitation was conducted using image algorithm as described in Materials and Methods. Each group included 3 to 4 mice. *, statistically significant differences from untreated mice (P < 0.05). D, effect of combination therapy depends on time of the treatment. B16F10 tumors were established in C57BL/6 mice. On day 11, mice received 5 × 106 activated Pmel T cells (T cells and T cells + paclitaxel group) or paclitaxel (12.5 mg/kg; paclitaxel + T cells group). Mice from T cells + paclitaxel groups received paclitaxel on day 14 and mice from paclitaxel + T cells group received T cells on day 16. Each group included 5 mice, mean ± SD are shown. Unt, untreated cells.
To determine the effects of chemotherapy on the level of the receptor in vivo, tumors were established in either syngenic mice (C57BL/6 for B6F10, BALB/c for 4T1) or nonobese diabetic/severe combined immunodeficient (NOD/SCID) or nude mice (human tumors). When tumors reached 1 cm in diameter mice were treated with doses of appropriate chemotherapy (paclitaxel for B16F10 and 4T1 tumors; doxorubicin for multiple myeloma tumors; cisplatin for lung carcinoma) that resulted in a substantial inhibition of tumor progression within 7 to 10 days after administration (data not shown). In all tumor models, significant (P < 0.01) upregulation of MPR was detected 48 hours after the injection of chemotherapeutic drugs. It remained elevated for another 24 hours and then returned to the pretreatment level within 5 days after injection (Fig. 1B and C and Supplementary Fig. S1).
We then asked whether the kinetics of MPR upregulation was relevant for the antitumor effects of combination therapy. Mice with established B6F10 melanoma were treated with paclitaxel alone, adoptive transfer of in vitro activated Pmel-1 CTLs that recognize gp100-derived epitope on B16F10 cells, or with the combination of these 2 therapies. Administration of either T cells or paclitaxel alone substantially delayed tumor growth, which however, resumed 1 week after the treatment (Fig. 1D). When paclitaxel was combined with T-cell therapy, a significant (P < 0.05) potentiating effect was observed. However, this effect was seen only if paclitaxel was administered 2 days after T cells. When paclitaxel was injected 5 days before T-cell administration, no increased antitumor effect was observed (Fig. 1D). Thus chemotherapy induced transient upregulation of MPR on tumor cells, which was directly associated with antitumor effect of combined chemoimmunotherapy.
The role of MPR in the antitumor effect of combination chemoimmunotherapy
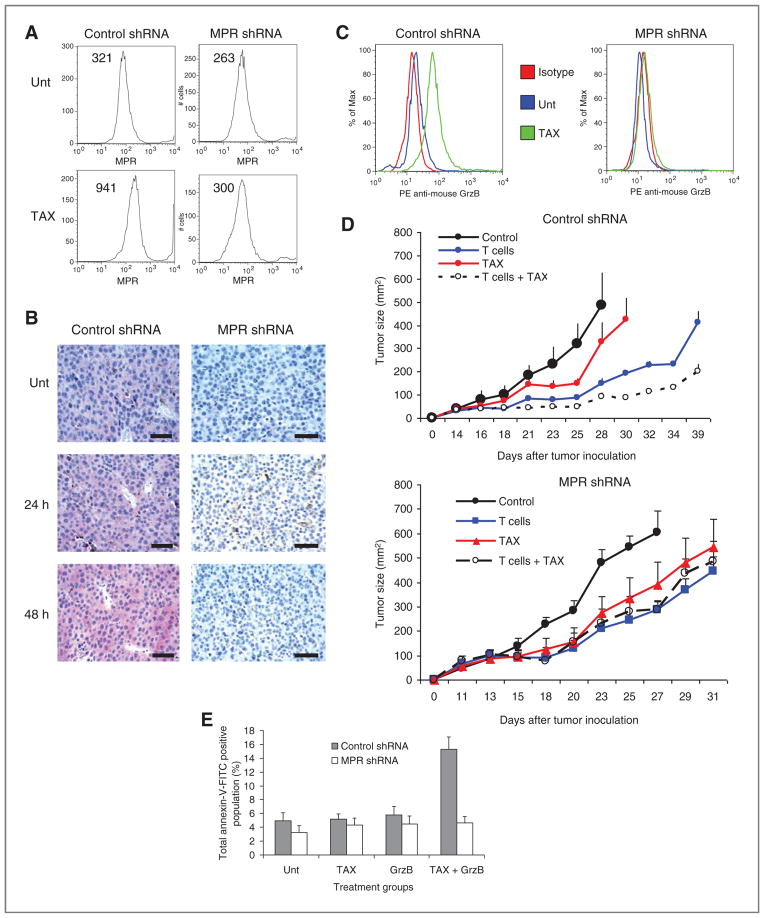
We generated a B16F10 tumor cell line with stable expression of MPR shRNA. In contrast to cells transfected with control shRNA, cells transfected with MPR shRNA did not upregulate MPR in response to paclitaxel treatment in vitro (Fig. 2A) or in vivo (Fig. 2B). Paclitaxel did not increase granzyme B penetration into cells expressing MPR shRNA (Fig. 2C). Treatment of mice bearing B16F10 tumors expressing control shRNA with the combination of paclitaxel and Pmel-1 CTL showed antitumor activity that was significantly (P < 0.05) higher than each therapy alone. This effect was not seen in mice bearing tumors expressing MPR shRNA (Fig. 2D).
Figure 2.
MPR is responsible for the antitumor effect of combined chemoimmunotherapy. B16F10 cells were transfected with control shRNA or MPR shRNA. A, effect of overnight treatment with 12.5 ng/mL paclitaxel (TAX) on expression of MPR on tumor cells surface. B, effect of paclitaxel (12.5 mg/kg) treatment of B16F10 tumor-bearing mice on expression of MPR in tumors. C, granzyme B (GrzB) penetration into tumor cells after overnight treatment of B16F10 cells with paclitaxel. D, treatment of mice with adoptive T-cell transfer (T cells), Paclitaxel, or combination as described in Fig. 1F. Each group included 5 mice. Mean ± SD are shown. E, downregulation of MPR abrogated granzyme B mediated killing of tumor cells pretreated with paclitaxel. Control shRNA and MPR shRNA B16F10 cells were treated with vehicle alone (Unt) or 12.5 ng/mL paclitaxel overnight followed by 6 hours treatment with 40 nmol/L recombinant mouse granzyme B. The percentage of Annexin-V positive cells from 3 conducted experiments is shown.
We tested the direct role of MPR in chemotherapy-inducible penetration of recombinant granzyme B to the tumor cells in vitro. As expected, treatment of control shRNA B16F10 with granzyme B during 6 hours did not result in induction of apoptosis. However, pretreatment of these cells with paclitaxel made them sensitive to the subsequent treatment with granzyme B. This effect was completely abrogated in MPR shRNA B16F10 cells (Fig. 2E).
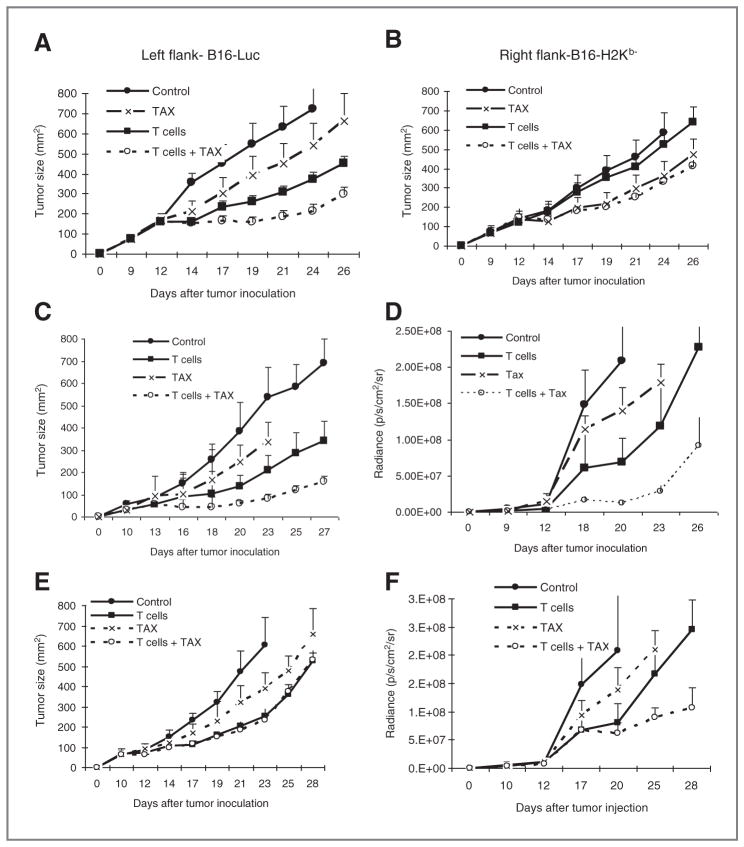
To test this hypothesis in vivo we used wild-type (H2Kb) B16F10 cells expressing luciferase (B16-Luc) and B16F10 H2Kb− cells. B16-H2Kb− cells were transfected with control or MPR shRNA. As effector cells, we used CTLs that recognize a Trp2180-188-derived peptide epitope expressed on B16F10 cells. As this epitope is recognized in the context of H2Kb, these CTLs do not kill B16-H2Kb− cells (data not shown). In this experimental system, we measured the tumor size of both B16-Luc and B16-H2Kb− tumors with calipers and independently monitored the growth of B16-Luc tumor using in vivo imaging.
First, we used control shRNA B16-H2Kb− cells. B16-Luc and B16-H2Kb− cells showed similar growth rates after subcutaneous (s.c.) injection into C57BL/6 mice (data not shown). To test the effect of therapy, B16-Luc and B16-H2Kb− tumors were established in the opposite flanks of the same mouse. When tumors became palpable, mice were split into 4 groups and treated with either paclitaxel or CTLs alone, no treatment or with the combination. Paclitaxel, at the selected dose, had a modest antitumor effect against both tumors, whereas Trp2 CTLs had antitumor effects only against wild-type B16-Luc cells (Fig. 3A) but not against B16-H2Kb− cells (Fig. 3B). Significant (P < 0.05) potentiation of the antitumor effects of combined paclitaxel and CTLs was observed only in the flank with B16-Luc tumor (Fig. 3A) but not with B16-H2Kb− tumors (Fig. 3B).
Figure 3.
MPR mediates a bystander effect of combination therapy. A and B, B16-Luc (H2Kb+) tumor cells (A) were established in the left flank and B16-H2Kb− in the right flank of the same mice (B). Mice were treated with Trp2-specific CD8+ T cells on days 10 and 16 followed by vaccinations with specific peptide on days 11 and 17. Paclitaxel (TAX) group and combination therapy group (T cells + paclitaxel) received paclitaxel (12.5 mg/kg) on days 13 and 22. Each group included 4 to 5 mice. Mean ± SD are shown. C–F, B16-luc tumor cells were mixed at 1:1 ratio (1.5 × 105 cells each) with either H2Kb− cells transfected with control shRNA (C and D) or H2Kb− cells transfected with MPR shRNA (E and F) and injected s.c. Mice were treated as described above. Tumor size was monitored using calipers (C and E) and by in vivo imaging (D and F). Each group included 4 to 5 mice. Mean ± SD are shown.
In the other sets of experiments, B16-Luc and B16-H2Kb− cells were mixed together at a 1:1 ratio and injected s.c. into mice. Mice with established tumors were then treated with paclitaxel and Trp2 CTLs either separately or in combination. Combining paclitaxel and CTLs resulted in a significantly (P < 0.05) greater antitumor effect than each of them separately. This was seen by monitoring tumor size with calipers (Fig. 3C) and by in vivo imaging (Fig. 3D and Supplementary Fig. S2). Similar experiments were conducted by mixing B16-Luc and B16-H2Kb− cells transfected with MPR shRNA. In contrast to described above results, potentiation of the antitumor effect of combination therapy was not detected when B16-Luc cells were mixed with B16-H2Kb−cells transfected with control shRNA (Fig. 3E). This effect was most likely because of the outgrowth of B16-H2Kb−cells as combination of paclitaxel and CTLs had potent antitumor activity against B16-Luc cells when these cells were monitored by in vivo imaging (Fig. 3F and Supplementary Fig. S2). These results indicate that chemotherapy and immunotherapy combined in vivo resulted in bystander killing of tumor cells without the need for antigen recognition and that this effect was mediated by MPR.
Mechanism of MPR upregulation by chemotherapy
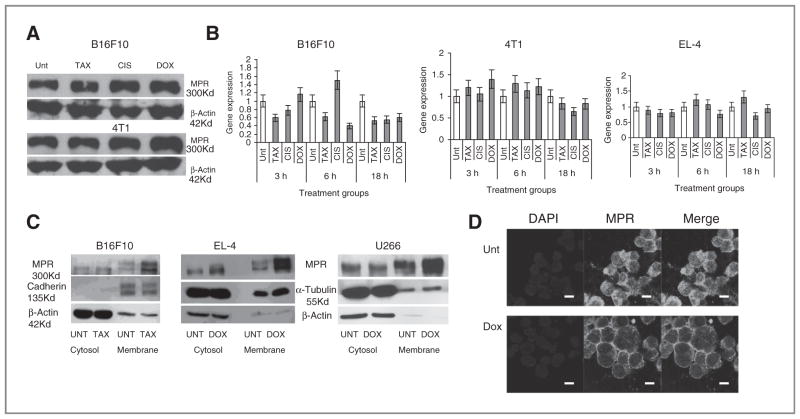
We then asked how chemotherapy could regulate MPR on tumor cells. We tested the possible regulation of MPR synthesis or degradation by chemotherapy agents. We did not observe the effect of paclitaxel, cisplatin, or doxorubicin on total amount of MPR protein by using Western blot (Fig. 4A) or flow cytometry after fixation and permeabilization of the cells (Supplementary Fig. S3). No effect of chemotherapy drugs on Mpr expression was found (Fig. 4B).
Figure 4.
The mechanism of MPR upregulation in tumor cells after chemotherapy. A and B, 4T1 or B16F10 tumor cells were treated overnight with paclitaxel (TAX) and U266 with doxorubicin (DOX). The amount of total protein was evaluated by Western blotting (A) and mpr mRNA by qRT-PCR (B). C, membrane and cytoplasmic fractions of tumor cells treated with paclitaxel or doxorubicin were isolated and MPR was detected by Western blotting. D, U266 were treated overnight with doxorubicin, fixed and stained with MPR specific antibody, and analyzed by confocal microscopy. Bar, 25 μm. Unt, untreated cells.
We asked whether MPR accumulation is limited to the cell membrane. In 3 different tumor cell lines treated with paclitaxel or doxorubicin, chemotherapy induced substantial accumulation of MPR only in a membrane fraction (Fig. 4C). To verify these findings we evaluated MPR expression in human multiple myeloma U266 cells treated with doxorubicin using confocal microscopy. Chemotherapy caused redistribution of MPR from a primarily cytoplasmic to a predominantly membrane localization of MPR (Fig. 4D). Similar results were obtained with mouse B16F10 cells treated with paclitaxel (Supplementary Fig. S4).
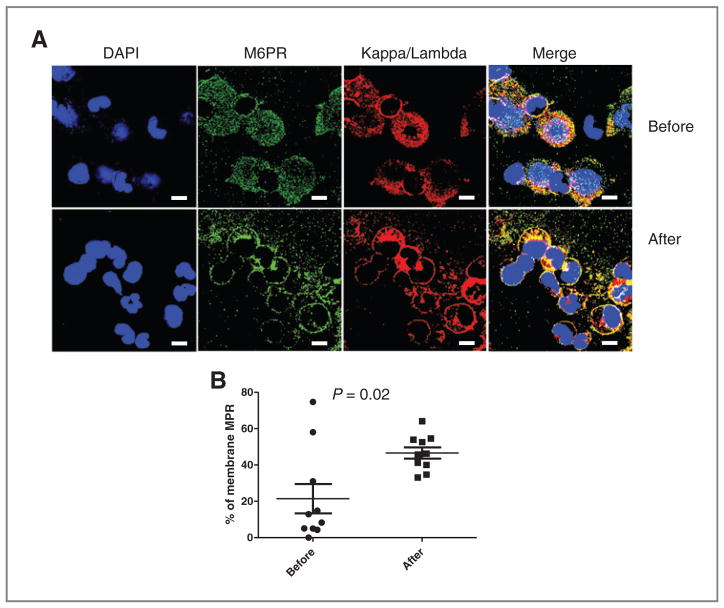
To determine whether similar effects could be observed in cancer patients, we used archived bone marrow samples obtained from patients with multiple myeloma treated on phase I trial of high-dose melphalan and topotecan followed by peripheral blood stem cell rescue. Bone marrow cells were collected from these patients before and after 3 days of high-dose chemotherapy. The type of immunoglobulin secreted by multiple myeloma for each patient has been determined; therefore, we could use appropriate kappa or lambda chain-specific antibodies to detect multiple myeloma cells. In addition, cells were stained with MPR-specific antibody. We assessed the proportion of multiple myeloma cells with predominantly membrane localization of MPR (Fig. 5A). Before treatment, only 20% of the multiple myeloma cells had such a characteristic, whereas after 3 days of high-dose chemotherapy this proportion increased to more than 50% of all multiple myeloma cells (P = 0.02; Fig. 5B).
Figure 5.
Effect of chemotherapy on MPR expression in patients with multiple myeloma. Paired samples of bone marrow aspirates from patients with multiple myeloma collected before and after 3 days of chemotherapy stained with the indicated kappa or lambda antibodies (depending on the types produced by multiple myeloma cells) and MPR antibody. A, typical example of staining. Bar, 50 μm. B, the proportion of cells with membrane MPR staining. Cumulative results from 10 samples is shown.
Autophagy as a mechanism of MPR accumulation on the cell membrane
How can chemotherapy induce accumulation of MPR on the cell surface? Autophagy is a common effect of chemotherapy on tumor or endothelial cells (28–31). It is a relatively rapid process and often associated with tumor cell survival from chemotherapy. The mechanism of accumulation of autophagosomes in tumor cells depends on type of chemotherapy used. We tested the possible role of autophagy in MPR upregulation. As expected, treatment of tumor cells with paclitaxel, cisplatin, or doxorubicin caused rapid induction of autophagy as determined by the appearance of autophagy-specific LC3 punctae in treated cells (Fig. 6A). For pharmacologic inhibition of autophagy we used 3MA, established specific inhibitor of autophagy. 3MA abrogated upregulation of MPR on the surface of U266 multiple myeloma cells treated with doxorubicin (Fig. 6B) or B16F10 cells treated with paclitaxel (Fig. 6C). 3MA also blocked the doxorubicin-inducible redistribution of MPR to the cell membrane (Fig. 6D). The cytotoxic activity of Pmel-1 CTLs against B16F10 target cells treated with or without chemotherapy was tested in a standard chromium release assay. Pretreatment of tumor cells with paclitaxel dramatically increased Pmel-1 mediated killing of target cells. In contrast, this effect was completely abrogated in the presence of 3MA (Fig. 6E).
Figure 6.
The link between autophagy and MPR expression in tumor cells. A, formation of LC3 punctae in tumor cells by overnight treatment with different drugs. Staining with LC3 specific antibody (red). Bar, 25 μm. B–D, MPR upregulation in B16F10 cell induced by paclitaxel (TAX; B) or in U266 cells induced by doxorubicin (DOX; C and D) was abrogated by inhibitor of autophagy 3MA. Staining of cells was evaluated by flow cytometry (B and C) and by confocal microscopy (D). A–D, each experiment was conducted at least 3 times. E, autophagy inhibitor 3MA abrogated combined cytotoxic effect of paclitaxel (12.5 ng/ mL, overnight treatment) and CTLs (activated Pmel T cells) against B16F10 tumor cells. Standard 51Cr-release assay was conducted in duplicates. Results of 2 experiments are shown (mean ± SD). Unt, untreated cells.
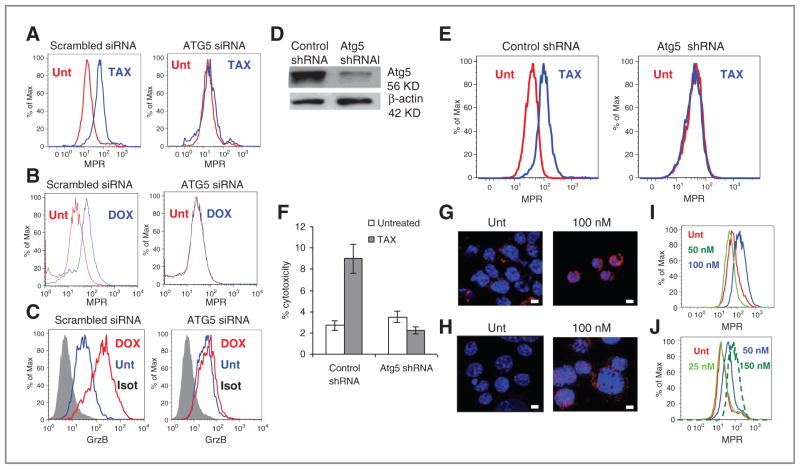
To further evaluate the role of autophagy in MPR upregulation, we downregulated expression of atg5, the gene critically important for induction of autophagosomes, using siRNA with 2 different siRNA (data not shown). Transfection of B16F10 cells with atg5 siRNA completely abrogated the paclitaxel-inducible increase in MPR presence on the cell surface (Fig. 7A). Transfection of atg5siRNA to U266 multiple myeloma cells abrogated doxorubicin inducible upregulation of MPR (Fig. 7B). It also completely abrogated granzyme B penetration to these cells (Fig. 7C). To verify these findings independently, we established melanoma B16F10 cell line with stable expression of atg5 shRNA. These cells have substantially lower level of Atg5 than B16F10 cells transfected with control shRNA (Fig. 7D). Downregulation of Atg5 abrogated paclitaxel-inducible upregulation of MPR on the surface of the tumor cells (Fig. 7E) and increased killing of tumor cells by specific CTLs (Fig. 7F).
Figure 7.
Autophagy causes upregulation of MPR in tumor cells. A, downregulation of atg5 in B16F10 cells using siRNA abrogated paclitaxel (TAX)-inducible upregulation of MPR. B, downregulation of atg5 in U266 cells using siRNA abrogated doxorubicin (DOX)-inducible upregulation of MPR. C, downregulation of atg5 in U266 cells using siRNA abrogated doxorubicin-inducible uptake of granzyme B (GrzB). A–C, typical examples are shown. Each experiment was conducted at least 3 times. D, downregulation of Atg5 in B16F10 cell line with stable transfection of atg5 shRNA. E, Effect of paclitaxel on upregulation of MPR on B16F10 tumor cells transfected with control or atg5 shRNA. F, downregulation of Atg5 in tumor cells abrogated paclitaxel-inducible upregulation of tumor-cell killing by CTLs in 4-hour chromium release assay. Target:effector ratio 1:12.5 is shown. Experiment was conducted in triplicates. G–J, U266 (G and I) or B16F10 (H and J) cells were treated overnight with 100 nmol/L rapamycin. Autophagy was evaluated by LC3 staining (bars, 25 μm; G and H) and MPR expression by flow cytometry (I and J). Two experiments with the same results were conducted. Unt, untreated cells.
To assess a direct role of autophagy on regulation of MPR expression on the cell surface we used rapamycin, a known inducer of autophagy (32). Autophagy (determined by LC3 punctae) was detected in U266 multiple myeloma (Fig. 7G) and B16F10 (Fig. 7H) cells treated with rapamycin at concentration of 100 nmol/L. At this and higher doses, rapamycin caused a substantial increase in MPR levels on the tumor cell surface (Fig. 7I and J).
Discussion
In this study, we tried to determine why the combination of chemotherapy with adoptive T-cell transfer resulted in an enhanced antitumor effect. This study was based on previous serendipitous findings obtained in a number of clinical trials demonstrating an unusually high rate of objective clinical responses observed in patients treated with conventional chemotherapy after failing cancer vaccines (6). These effects were obtained with different chemotherapeutic drugs and different types of cancer vaccines suggesting that there was some common mechanism involved. Moreover, in all those cases, conventional doses of chemotherapy were used, which are known to inhibit immune responses. Therefore, although the ability of chemotherapy to induce immunogeneic tumor cell death has been described (33, 34), this mechanism was unlikely involved in this case. Recently, we showed in experiments in vitro a possible role of MPR in this process. Whether this mechanism is indeed operational in vivo remained unclear. More importantly, the role of this process in mediating the antitumor effect of combination therapy as well as the mechanism of MPR upregulation on tumor cells was unknown. To address these questions, we used different tumor models and chemotherapeutic drugs with different mechanism of action. The doses of the drugs were selected to impact on tumor progression. Most of the experiments with combined treatment were conducted with paclitaxel. Previous studies have shown that at selected doses, repeated injections of paclitaxel are immune suppressive (35).
As immune therapy, we used the adoptive transfer of antigen-specific T cells, a promising new method of treatment that has shown antitumor effects in mice and patients (36–38). We found that chemotherapy caused dramatic, albeit only transient upregulation of MPR on tumor cells in vivo. This effect was seen in every tumor model tested and with all drugs used. Importantly, the effect of combined treatment was seen only when chemotherapy was given within a window of time during which increased level of MPR was observed on tumor cells. After the MPR level was normalized, addition of adoptive T-cell transfer did not induce any further therapeutic benefit. This result suggested that the effect of combination therapy depended on upregulation of MPR. To test this hypothesis directly, we inhibited MPR expression on tumor cells using shRNA and found that the antitumor effect of combination chemoimmunotherapy was abrogated in mice bearing MPR-deficient tumor cells.
We suggested that if MPR did indeed mediate the antitumor effect of CTLs in combination with chemotherapy, then tumor cells not expressing specific antigens, and thus not recognized by CTLs would not be sensitive to the effect of combination therapy. Our data have shown that no antitumor effect of T-cell transfer was seen in mice bearing tumors lacking one of MHC class I allele (H2Kb), which binds the specific peptide (Trp2180-188) that is recognized by CTLs. Not surprisingly, in that tumor, combination therapy did not provide any additional benefit over the effect of chemotherapy alone. However, when wild-type and H2Kb− tumor cells were mixed together the effect of combined chemoimmunotherapy was prominent. Apparently, activated CTLs were able to kill not only wild-type tumor cells that expressed specific antigen but also those that could not be recognized by CTLs (H2Kb− cells). This effect was abrogated when MPR deficient H2Kb− tumor cells were mixed with wild-type tumor cells. These data strongly support the concept that in combination with chemotherapy, activated CTLs can release granzyme B, which is able to bind MPR on neighboring tumor cells regardless of whether those cells express MHC class I or not.
The other main question we addressed in this study was how chemotherapy can upregulate MPR expression on the surface of tumor cells. Our data indicate that chemotherapy did not induce MPR synthesis and did not inhibit its degradation. Instead, we observed redistribution of MPR within the cells. Normally more than 90% of total MPR is localized inside the cells in different compartments (endosomes and TGN; ref. 39). After chemotherapy, large amount of MPR was localized on the cell membrane. This might account for stronger immunohistochemistry staining of tumor tissues after treatment with chemotherapy (MPR localized on cell surface is more accessible for antibodies and thus is better stained than MPR localized in intracellular compartments) and could explain the increased uptake of granzyme B by tumor cells after interaction with CTLs observed in our previous study (18).
What could cause redistribution of the MPR in the cell? In recent years, increasing evidence has accumulated about the importance of autophagy in cancer treatment (40–42). Autophagy is a reversible process that can contribute both to tumor cell death and survival. This catabolic pathway is initiated by the formation of a phagophore around cytoplasmic organelles and/or some portion of the cytosol. The enclosed material is sequestered in a vacuole lined by 2 membranes called the autophagosome. Autophagosomes then undergo fusion with either endosomes or lysosomes. The mechanism of autophagosome formation depends on the type of chemotherapeutic drug. In our study, paclitaxel, doxorubicin, and cisplatin caused rapid induction of autophagy in tumor cells, which was associated with upregulation of MPR. Blockade of autophagy with either its inhibitor 3MA or by downregulating atg5 resulted in abrogation of chemotherapy-induced upregulation of MPR on the tumor cell surface. How exactly this may happen is not entirely clear. We propose that MPR is redirected to autophagosomes either as part of clathrin-coated vesicles, or as part of fusion of autophagosomes with endosomes. In both cases, low pH in autophagosomes results in release of the MPR cargo followed by shuttle of the receptor to the surface. The detailed mechanism of this effect needs to be clarified.
Recently Michaud and colleagues showed that autophagy was dispensable for chemotherapy-induced cell death but required for its immunogenicity. In response to chemotherapy, autophagy-competent, but not autophagy-deficient cancers attracted dendritic cells and T lymphocytes into the tumor bed. This effect was mediated via ATP (43). The issue of the relationship between autophagy and tumor immunity requires further elucidation as there is evidence that autophagy can limit immmune-mediated cytotoxicity (44) and that autophagy-deficient tumors may be rare (45).
Our study presents a novel concept relating to the interaction between CTLs and tumor cells undergoing autophagy that can be clinically exploited not only in the setting of chemotherapy but also radiation therapy, as well as other treatments that cause autophagy of tumor cells. Our data show that combining chemotherapy and immunotherapy as a front line therapy in patients with advanced cancer has a strong rationale. However, it needs to be carefully timed and may be monitored using MPR expression.
Supplementary Material
Figure S3. Redistribution of MPR to the cell surface after chemotherapy. MPR levels in fixed cells. 4T1 and B16F10 cell lines were treated with 12.5nM TAX for 16h and labeled with MPR antibody as described earlier (Ramakrishnan et al., 2010). A portion of the cells was fixed with 2% paraformaldehye for 20 min at room temperature. The cells were then stained for MPR expression. Appropriate untreated and isotype controls were used for the experiments. The expression of MPR was evaluated in viable and fixed cells by BD LSR-II flow cytometer. Dead cells were discriminated from the live population by either DAPI stain or Live/Dead Fixable Dead cell stain kit (Invitrogen). Typical example of three performed experiments is shown
Figure S4. Redistribution of MPR to the cell surface after chemotherapy. B16F10 cells were grown on slides coated with poly-D-Lysine and treated with TAX 12.5nM for 16h. The slides were washed, fixed with 4% paraformaldehyde and blocked with 5% BSA for 30 min. Cells were labeled with primary antibody for MPR followed by the anti-rabbit MPR (red fluorescence). For labeling of TNG, goat anti rabbit giantin (Abcam) was used (green fluorescence). The slides were covered with Vectastain DAPI. Micrographs of B16 F10 cells were taken with a Leica TCS SP5 AOBS laser scanning confocal microscope through a 63X/1.40NA Plan Apochromat oil immersion objective lens (Leica Microsystems, Germany). 405 nm and 633 nm diode lasers, and an argon tunable 488nm laser lines were applied to excite the samples. An acoustic optical beam splitter was used to minimize crosstalk between fluorochromes. Over twenty 500 nm image z-sections for each sample were captured with photomultiplier detectors and prepared with the LAS AF software version 2.1.0 (Leica Microsystems, Germany). One section is shown.
Acknowledgments
Grant Support
This work was supported in part by a grant from Donald A. Adams Comprehensive Melanoma Research Center to D.I. Gabrilovich, NIH grant R01CA103921 to E. Celis, and by the analytic microscopy and flow cytometry cores of H. Lee Moffitt Cancer Center.
Footnotes
Disclosure of Potential Conflicts of Interest
E. Celis has ownership interest (including patents) in Patent Application for Vaccine Technology. No potential conflicts of interest were disclosed by the other authors.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Authors’ Contributions
Conception and design: C. Huang, E. Celis, D.I. Gabrilovich
Development of methodology: R. Ramakrishnan, C. huang, H.-I. Cho, E. Celis, D.I. Gabrilovich
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): C. Huang, H.-I. Cho, M. Lloyd, J. Johnson, S. Altiok, D. Sullivan, J. Weber
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): R. Ramakrishnan, C. Huang, M. Lloyd, J. Johnson, D.I. Gabrilovich
Writing, review, and/or revision of the manuscript: R. Ramakrishnan, M. Lloyd, D. Sullivan, J. Weber, D.I. Gabrilovich
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): R. Ramakrishnan, C. huang, H.-I. Cho, M. Lloyd, J. Johnson, D.I. Gabrilovich
Study supervision: E. Celis, D.I. Gabrilovich
References
- 1.Gribben JG, Ryan DP, Boyajian R, Urban RG, Hedley ML, Beach K, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–6. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12(3 Pt 1):878–87. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 3.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–9. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–82. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–26. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57:1523–9. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 9.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–63. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–44. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69:6987–94. doi: 10.1158/0008-5472.CAN-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergmann-Leitner ES, Abrams SI. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8+ cytotoxic T lymphocytes. Cancer Immunol Immunother. 2001;50:445–55. doi: 10.1007/s002620100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–64. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 14.Behl D, Porrata LF, Markovic SN, Letendre L, Pruthi RK, Hook CC, et al. Absolute lymphocyte count recovery after induction chemotherapy predicts superior survival in acute myelogenous leukemia. Leukemia. 2006;20:29–34. doi: 10.1038/sj.leu.2404032. [DOI] [PubMed] [Google Scholar]
- 15.Liseth K, Ersvaer E, Hervig T, Bruserud O. Combination of intensive chemotherapy and anticancer vaccines in the treatment of human malignancies: the hematological experience. J Biomed Biotechnol. 2010;2010:692097. doi: 10.1155/2010/692097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor–secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 17.Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol. 2007;25:5426–34. doi: 10.1200/JCO.2007.12.0253. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–24. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–43. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh P, Kornfeld S. The GGA proteins: key players in protein sorting at the trans-Golgi network. Eur J Cell Biol. 2004;83:257–62. doi: 10.1078/0171-9335-00374. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh P, Dahms N, Kornfeld S. Mannose 6-phosphate receptros: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–12. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 22.Motyka B, Korbutt G, Pinkoski MJ, Heibein JA, Caputo A, Hobman M, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000;103:491–500. doi: 10.1016/s0092-8674(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 23.Trapani JA, Sutton VR, Thia KY, Li YQ, Froelich CJ, Jans DA, et al. A clathrin/dynamin- and mannose-6-phosphate receptor-independent pathway for granzyme B-induced cell death. J Cell Biol. 2003;160:223–33. doi: 10.1083/jcb.200210150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veugelers K, Motyka B, Goping IS, Shostak I, Sawchuk T, Bleackley RC. Granule-mediated killing by granzyme B and perforin requires a mannose 6-phosphate receptor and is augmented by cell surface heparan sulfate. Mol Biol Cell. 2006;17:623–33. doi: 10.1091/mbc.E05-07-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dressel R, Raja SM, Honing S, Seidler T, Froelich CJ, von Figura K, et al. Granzyme-mediated cytotoxicity does not involve the mannose 6-phosphate receptors on target cells. J Biol Chem. 2004;279:20200–10. doi: 10.1074/jbc.M313108200. [DOI] [PubMed] [Google Scholar]
- 26.Bolitho P, Voskoboinik I, Trapani JA, Smyth MJ. Apoptosis induced by the lymphocyte effector molecule perforin. Curr Opin Immunol. 2007;19:339–47. doi: 10.1016/j.coi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Cho HI, Lee YR, Celis E. Interferon gamma limits the effectiveness of melanoma peptide vaccines. Blood. 2011;117:135–44. doi: 10.1182/blood-2010-08-298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notte A, Leclere L, Michiels C. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem Pharmacol. 2011;82:427–34. doi: 10.1016/j.bcp.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 29.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–16. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–66. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi S, Yamamoto A, You F, Yamashita K, Ikegame Y, Tawada M, et al. The stent-eluting drugs sirolimus and paclitaxel suppress healing of the endothelium by induction of autophagy. Am J Pathol. 2009;175:2226–34. doi: 10.2353/ajpath.2009.090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciuffreda L, Di Sanza C, Incani UC, Milella M. The mTOR pathway: a new target in cancer therapy. Curr Cancer Drug Targets. 2010;10:484–95. doi: 10.2174/156800910791517172. [DOI] [PubMed] [Google Scholar]
- 33.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–60. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 34.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–94. [PubMed] [Google Scholar]
- 36.Turcotte S, Rosenberg SA. Immunotherapy for metastatic solid cancers. Adv Surg. 2011;45:341–60. doi: 10.1016/j.yasu.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17:1664–73. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- 38.Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet? Immunol Rev. 2011;239:27–44. doi: 10.1111/j.1600-065X.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown J, Jones EY, Forbes BE. Interactions of IGF-II with the IGF2R/cation-independent mannose-6-phosphate receptor mechanism and biological outcomes. Vitam Horm. 2009;80:699–719. doi: 10.1016/S0083-6729(08)00625-0. [DOI] [PubMed] [Google Scholar]
- 40.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 41.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–9. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 43.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 44.Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P, et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res. 2011;71:5976–86. doi: 10.1158/0008-5472.CAN-11-1094. [DOI] [PubMed] [Google Scholar]
- 45.Amaravadi R. Autophagy in tumor immunity. Science. 2011;334:1501–2. doi: 10.1126/science.1216428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S3. Redistribution of MPR to the cell surface after chemotherapy. MPR levels in fixed cells. 4T1 and B16F10 cell lines were treated with 12.5nM TAX for 16h and labeled with MPR antibody as described earlier (Ramakrishnan et al., 2010). A portion of the cells was fixed with 2% paraformaldehye for 20 min at room temperature. The cells were then stained for MPR expression. Appropriate untreated and isotype controls were used for the experiments. The expression of MPR was evaluated in viable and fixed cells by BD LSR-II flow cytometer. Dead cells were discriminated from the live population by either DAPI stain or Live/Dead Fixable Dead cell stain kit (Invitrogen). Typical example of three performed experiments is shown
Figure S4. Redistribution of MPR to the cell surface after chemotherapy. B16F10 cells were grown on slides coated with poly-D-Lysine and treated with TAX 12.5nM for 16h. The slides were washed, fixed with 4% paraformaldehyde and blocked with 5% BSA for 30 min. Cells were labeled with primary antibody for MPR followed by the anti-rabbit MPR (red fluorescence). For labeling of TNG, goat anti rabbit giantin (Abcam) was used (green fluorescence). The slides were covered with Vectastain DAPI. Micrographs of B16 F10 cells were taken with a Leica TCS SP5 AOBS laser scanning confocal microscope through a 63X/1.40NA Plan Apochromat oil immersion objective lens (Leica Microsystems, Germany). 405 nm and 633 nm diode lasers, and an argon tunable 488nm laser lines were applied to excite the samples. An acoustic optical beam splitter was used to minimize crosstalk between fluorochromes. Over twenty 500 nm image z-sections for each sample were captured with photomultiplier detectors and prepared with the LAS AF software version 2.1.0 (Leica Microsystems, Germany). One section is shown.