Abstract
β-defensin peptides are a large family of antimicrobial peptides. Although they kill microbes in vitro and interact with immune cells, the precise role of these genes in vivo remains uncertain. Despite their inducible presence at mucosal surfaces, their main site of expression is the epididymis. Recent evidence suggests that a major function of these peptides is in sperm maturation. In addition to previous work suggesting this, work at the MRC Human Genetics Unit, Edinburgh, has shown that homozygous deletion of a cluster of nine β-defensin genes in the mouse results in profound male sterility. The spermatozoa derived from the mutants had reduced motility and increased fragility. Epididymal spermatozoa isolated from the cauda region of the homozygous mutants demonstrated precocious capacitation and increased spontaneous acrosome reactions compared with those from wild-types. Despite this, these mutant spermatozoa had reduced ability to bind to the zona pellucida of oocytes. Ultrastructural examination revealed a disintegration of the microtubule structure of mutant-derived spermatozoa isolated from the epididymal cauda region, but not from the caput. Consistent with premature acrosome reaction and hyperactivation, spermatozoa from mutant animals had significantly increased intracellular calcium content. This work demonstrates that in vivo β-defensins are essential for successful sperm maturation, and that their disruption alters intracellular calcium levels, which most likely leads to premature activation and spontaneous acrosome reactions that result in hyperactivation and loss of microtubule structure of the axoneme. Determining which of the nine genes are responsible for the phenotype and the relevance to human sperm function is important for future work on male infertility.
Keywords: acrosome reaction, antimicrobial, capacitation, epididymis, sperm, β-defensins
BACKGROUND TO β-DEFENSINS
β-defensins are cationic peptides with a canonical six cysteines in their mature secreted form, which were first isolated as antimicrobials, and their presumed function is in host defense. The β-defensin gene family consists of 40 family members at five gene loci in humans and more than 50 genes over four loci in the mouse.1,2 The main cluster is on chromosome 8 in both humans and mice with 10 and 31 β-defensin genes, respectively. In humans, seven of the chromosome 8 genes lie at two distinct loci approximately 5 Mb apart as highly copy number variable (CNV) clusters, which vary between 2 and 7 copies per genome.3 It is, therefore, possible that phenotypic change may be present owing to reduced or increased copy number. Increased copy number above the mean diploid number of 4 has been associated with an increased risk of psoriasis.4 It is evident that the evolutionary history of this gene family is complex with evidence for both rapid positive as well as negative selection.5 Despite recent evidence of an increasing functional repertoire of β-defensin peptides, which includes involvement in pigmentation, immune cell attraction and immunomodulation, the physiological function of mammalian β-defensins in vivo remains uncertain.
Previous work on the importance of β-defensins in sperm function
Interestingly, β-defensins are highly expressed under normal conditions in different segments of the epididymal epithelium2,6,7,8,9 and are likely to be secreted into the lumen, and some have been shown to be present on the plasma membrane of sperm cells.10,11,12,13,14 It seems likely therefore that they are involved in reproductive function, and a few studies over the last decade have suggested that β-defensins influence sperm maturation.
The rat β-defensin Bin1b (SPAG11 or EP2) has been shown to induce immature and immotile spermatozoa to become progressively motile in vitro.8 In addition, the β-defensin DEFB126 on chromosome 20 has recently been linked to the ability of human spermatozoa to penetrate hyaluronic acid gel (which mimics the consistency of female cervical secretions). Men homozygous for a frameshift mutation in DEFB126 are not infertile but have reduced the chance of paternity in the first year.12 DEFB126 is quite different from other β-defensins, as it has an extensive C-terminal tail containing O-linked glycosylation sites that are not seen in other defensins. It is presumed that this glycosylation is important for its function. Additionally, in the rat incomplete knockdown of Defb15 suggests that this peptide influences sperm motility, but apparently not the capacitation process or the acrosome reaction (AR).13 Most recently low levels of human β-defensin 1 (hBD1) have been found in spermatozoa from infertile men with low sperm motility.10 This work also shows that the affected spermatozoa have a defect in bacteriocidal activity, and this can be corrected by addition of exogenous peptide.
GENERATION OF A β-DEFENSIN GENE KNOCKOUT IN THE MOUSE
The mouse is a good model of certain aspects of innate immunity although many of the multigene families have species- or environment-specific clades, and the β-defensin gene family is no exception.1,15 Some of the human defensin genes have single-gene orthologues in the mouse whereas some have several paralogues. Single-gene deletion can result in an obscure phenotype, as functional redundancy in gene families can play a significant role in obscuring functional roles. Some years ago my laboratory. and others created mice that were deleted for Defb1 (the mouse orthologue of the gene for hBD1)16 and the homozygous animals had a subtle gross phenotype: a defect in bacterial clearance from the airway and an increased number of Staphylococcal species in the bladder.17,18 The male mice were robustly fertile (unpublished).
In order to address the redundancy issue and ascertain in vivo function, my laboratory. has gone on to use gene targeting and lox/cre MICER (Mutagenic Insertion and Chromosome Engineering Resource) technology to delete selectively, the β-defensin gene clusters in the mouse.19,20,21 Initially, we deleted nine genes from the main 31 β-defensin gene cluster on chromosome 8 (peptide sequences of the deleted genes are shown in Table 1). These were Defb1, Defb50, Defb2, Defb10, Defb9, Defb11, Defb15, Defb35, and Defb13, which are the nine most telomeric genes of the cluster found adjacent to the intestinal α-defensin (cryptdins) genes. Defb1, Defb15, Defb35 and Defb13 are orthologous to the human genes DEFB1, DEFB106, DEFB105 and DEFB107, respectively, but Defb2, 10, 9, 11 (closely related paralogues) and Defb50 are in murine-restricted clades.5 All nine deleted genes and their human orthologues are most strongly expressed in the male reproductive tract.22 The homozygous mice produced from the ES cell lines (official allele name Del(chr8Defb1-Defb13)1JrDo but abbreviated here to DefbΔ9), had no obvious phenotype, although PCR analyses on genomic DNA or RT-PCR on cDNA synthesized from RNA from epididymides demonstrated the presence and expression of the nine deleted defensin genes in both heterozygous and wild-type mice, but their absence from homozygous mutant animals. Importantly, genes that are expressed in the epididymis and adjacent to, but outside the deletion (including Bin1b and Defb33), were not altered in their expression level. We did not find any increase in inflammatory cytokines in sera, and the mutant animals all had a normal body weight. Genotypic classes from heterozygous crosses were all in the expected 1:2:1 Mendelian ratio.
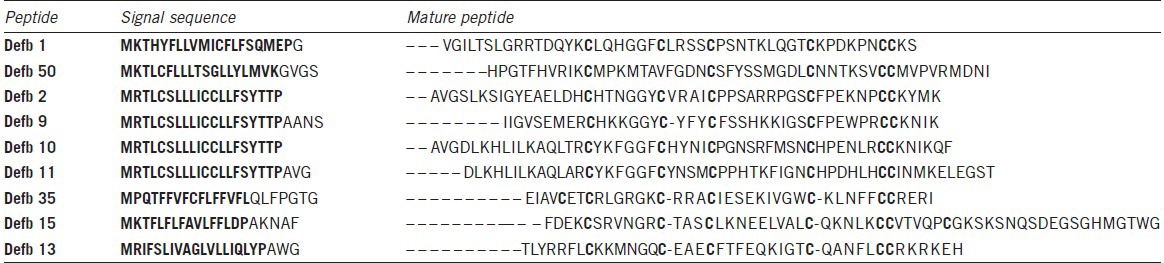
Table 1.
Amino acid sequence in single letter format, of the β-defensin peptides encoded by the genes deleted in the DefbΔ9/DefbΔ9 mice. The cysteine residues are emboldened in the mature peptide and sequences are lined up for best fit with dashes indicating where spaces have been introduced to facilitate this. The first exon of these two exon genes is in bold while the second exon is not. Signal sequence cleaved predicted by ExPASy proteomics tool
The homozygous deletion mice did, however, have an increased level of bacteria in urine extracted from the bladder. This supported the work that we had published previously on the Defb1 KO mice; Defb1 being one of the genes deleted in the defensin cluster KO mice.18β-defensins are classically known as antimicrobial peptides, so we tested the mature peptides in vitro against a Gram-negative bacterium (Pseudomonas aeruginosa, PAO1); all the peptides except Defb15 were strong antimicrobials in either oxidized or reduced form. The active antimicrobials include Defb50, which does not have the canonical six cysteines (the second cysteine of the motif is missing)(see Table 1). Defb50 has poor antimicrobial activity in its oxidized form, but even this improves under reduced conditions. These results support recent work by Schroeder et al. which suggests that some β-defensins display improved activity following reduction.23 Despite deletion of the expression of these antimicrobials from the homozygous mice, there was no indication that the mutant mice had increased inflammatory profile under normal specific pathogen-free animal housing conditions.
INFERTILITY PHENOTYPE OF MUTANT DEFBΔ09/DEFBΔ09 MICE
The mice that were initially produced as heterozygotes from chimeric animals derived from blastocysts injected with the ES cell gene-targeted lines and mated to C57Bl/6 females and were then inter-crossed to generate homozygous deletion mice. These mutant animals contained a high contribution of genetic background from the 129/Ola E14 ES cells, and the homozygous males at this point were fertile but showed a reduced ability to impregnate wild-type CD1 females. The male deletion mice sired equivalent numbers of litters but a low number of pups per litter. When the mice were inbred to four backcross generations onto C57Bl/6, the male homozygotes generated were completely infertile although homozygous females had normal fertility.
DefbΔ9/DefbΔ9 spermatozoa are fragile
In an effort to ascertain if the defect in fertility was due to sperm production, sperm numbers released from excised cauda region of the epididymis were assessed, but they were not significantly different between mutants males and wild-type C57Bl/6 male mice. In addition, tissue sections along the length of the epididymis were histologically normal, with evidence of comparable numbers of spermatozoa in the lumen. However, we did observe that isolated spermatozoa dropped onto a microscope slide were more fragile, as the number of headless tails counted was significantly higher in the mutants than in the wild-type animals.
DefbΔ9/DefbΔ9 spermatozoa have reduced motility and spontaneous acrosome reactions
In collaboration with Prof. Chris Barratt (University of Dundee), we used computer-assisted sperm analysis to determine the total and progressive motility of the spermatozoa from mutant and wild-type littermates. The sperm cells were very gently isolated from the cauda region of excised tissue to reduce any trauma to them, but nevertheless those from the mutants had a very obvious and significantly lower percentage of progressive motility both before capacitation, at zero time, and after capacitation induction, at 90 min. Further work demonstrated that the deletion mouse-derived spermatozoa had a higher percentage of spontaneous acrosome reactions (ARs) at time 0 and also at 90 min. The presence of an intact acrosome was measured by several different techniques. The first was the ability of spermatozoa to bind a fluorescently-labelled Pisum sativum (PSA) lectin that interacts with to the glycocalyx carbohydrate molecules of the acrosome. Secondly, an antibody to zonadhesin was used to demonstrate if binding was increased owing to loss of the acrosome revealing the zonadhesin epitope that binds the antibody. Finally, acrosomal integrity following Coomassie Blue G250 staining of fixed sperm cells was determined since acrosome-reacted spermatozoa lack G250 staining. By all these criteria, the spermatozoa from the mutant males had significantly increased level of spontaneous ARs. The level present upon isolation was maximal, as it appeared to be equivalent to the level evident in wild-type mouse sperm incubated for 90 min in capacitation medium.
Capacitation is the process by which spermatozoa in close proximity to the egg in the female reproductive tract will undergo physiological and membrane changes that enable fertilization of the oocyte. No direct procedure is available to determine capacitation status, but AR induction provides information on the rate of capacitation. Therefore, the extent of sperm capacitation can be evaluated indirectly by measuring the number of cells without an acrosome (using PSA binding to indicate acrosomal presence) following induction of the AR, as only capacitated cells can undergo this process. Capacitation of spermatozoa isolated from the cauda epididymidis was induced by the calcium ionophore A23187. The DefbΔ9/DefbΔ9-derived spermatozoa displayed the maximal percentage of capacitation immediately after exposure to the drug. This level did not increase after 90 min, whereas the status of sperm capacitation for wild-type spermatozoa increased from 6% to 23% over this same period.
DefbΔ9/DefbΔ9 sperm cannot bind to unfertilized eggs
As the mutant-derived spermatozoa were both acrosome reacted and capacitated, we tested if they were able to fertilize eggs by using a sperm-egg binding assay with wild-type eggs. The number of wild-type spermatozoa that could bind to the cumulus-free eggs from superovulated CD1 females was around 20 per egg after 45 min, but only negligible numbers of mutant spermatozoa were bound. Recent studies in the mouse have shown that spermatozoa that have undergone the AR can penetrate the zona pellucida (ZP), although this is not an efficient process,24,25 and that spermatozoa from several mouse KOs cannot strongly bind to the ZP and yet are still able to fertilize oocytes.26,27
DefbΔ9/DefbΔ9-derived spermatozoa have disordered microtubular structures
When ultrastructural analyses were made with transmission electron microscopy on spermatozoa released from the cauda epididymidis, an abnormally high number of cells with disruption of the classic 9 + 2 microtubule arrangement in the axoneme of spermatozoa from the mutants was observed compared with spermatozoa from wild-type littermates (41% for −/− vs 4% for +/+) (see Figure 1). We also analyzed tissue samples from the testes and caput and cauda regions and examined the structure of the sperm tails still within these organs so as to avoid artefacts producing damage due to processing. These analyses also revealed that spermatozoa present in the cauda (but not the caput or testes) of DefbΔ9/DefbΔ9 mutants showed an increase in the disruption of the microtubule structure, where the 9 + 2 arrangement was disintegrated. It may be of note that a very similar microtubular disruption phenotype is also seen in spermatozoa of mice with deletion of the group III secreted phospholipase A2(sPLA2-III), and as in the DefbΔ9 mutants, this is only present in sperm cells isolated from the cauda.28 Interestingly, secreted phospholipase A2 enzymes have a cysteine-rich structure and, like defensins, exhibit antimicrobial activity.
Figure 1.
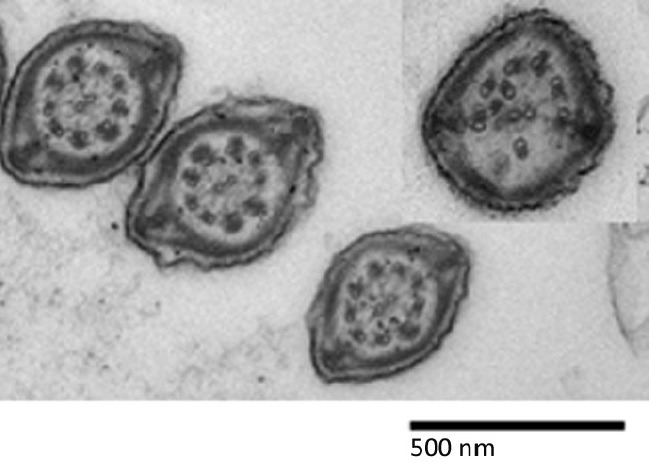
Transmission Electron Micrograph of characteristic spermatozoa from the cauda of a wild-type (+/+) and insert showing spermatozoa from DefbΔ9/DefbΔ9 (−/−) littermate.
When we measured the level of intracellular calcium in the mutant spermatozoa isolated from the cauda of the homozygous DefbΔ9/DefbΔ9 males, we found that it was significantly higher than that of wild-type spermatozoa under the same conditions. Thus, in the absence of the deleted β-defensins, there is a significant increase in intracellular calcium, and the likely consequence of this is an increase in spontaneous AR and microtubule disruption (possibly caused by hyperactivation). Normal spermatozoa may be able to withstand premature activation,29 but the mutant phenotype is reminiscent of demembranated sperm cells that have undergone hyperactivation following capacitation. In this situation, spermatozoa that are stimulated with calcium to increase tail movement can display disintegration of the axonemal filaments.30 The mutant defensin-deleted spermatozoa may have such a fragile membrane that the microtubule structure is subject to disorganization.
The fact that defensins have diverse receptor-binding activity may explain the increase in calcium seen in the mutant sperm cells. It is possible that normally one or more of the defensins can block a calcium channel such as CatSper. Indeed, the defensin-like molecule MsDef1 from Alfalfa seeds has been shown to have the ability to block mammalian L-type calcium channel activity.31 There is evidence to suggest that seminal fluid has components that suppress calcium transport activity until the membrane remodelling occurs during capacitation when the spermatozoa are in an appropriate region of the female reproductive tract.32 Perhaps β-defensins normally in the glycocalyx are part of this inhibitory process, protecting against inappropriate activation of spermatozoa in the epididymis, a site where sperm cells are mature but not appropriately located for fertilization.
UNANSWERED QUESTIONS REMAIN
These data show a clear function of one or more of the β-defensins in the deleted cluster being essential for sperm function, but it is not known if the sterility of the male reproductive tract is compromised. Is loss of genes with in vitro antimicrobial function important in vivo for infection defense? What of the immunomodulatory activity of defensins: is this of relevance in the epididymis? At the moment, we do not know which of the nine defensins genes are responsible for the unique fertility phenotype. It is suggested that epididymal maturation of sperm cells more likely occurs in the caput or corpus of the epididymis rather than the cauda.8 The defensins present in the deletion have been shown to be present in distinct regions of the epididymis. Defb15, 35 and 13 are expressed in the upper region (segments 1, 5 and 6, respectively), whereas Defb2, 9, 10, 11 and 50 expression is focused in segment 8 of the cauda region, while Defb1 is expressed in the penultimate segment of the epididymis.
Using mass spectrometry, we have only been able so far to detect Defb2, Defb11, and Defb15 on isolated wild-type cauda spermatozoa. Rat Defb15 has been reported to bind to the acrosomal region of caput sperm cells, and incomplete knockdown results in rats with reduced sperm motility, but no defect in capacitation or AR.13 Interestingly, Defb15 has an extended carboxyl tail containing an extra cysteine and six potential serine or threonine residues that would support O-linked glycosylation. O-linked glycosylation is considered a key feature of the function of DEFB126 in human spermatozoa, and it also seems to affect the ability of spermatozoa to move in a viscous hyaluronic acid used as a cervical mucus substitute. There is a paralogue of Defb15 not within the deleted region, and this gene (Defb34) is only three amino acids different from Defb15 in the mature segment of the peptide, which may make Defb15 a less likely candidate to affect spermatozoa.
It may be that deletion of a single-gene might not demonstrate a strong enough phenotype for it to be easily recognized. The three human orthologues to Defb15, Defb35 and Defb13 (DEFB106, DEFB105 and DEFB107, respectively) are on the chromosome 8 p23.1 highly copy number-variable block. In primates, protection against loss of fertility from mutation might be a selective advantage that allows the increase in copy number of these genes to be maintained. We have systematically knocked out the genes in the deletion using Crispr/Cas9 technology of pronuclear injection into C57Bl/6 eggs, and determination of fertility and sperm phenotype in these single-gene KOs is currently ongoing.
CONCLUDING REMARKS
For the first time, we show here that β-defensin antimicrobial peptide genes have a profound effect on sperm function in vivo, and this is manifest in the DefbΔ9/DefbΔ9 mice by increased intracellular calcium, precocious capacitation, increased spontaneous ARs, and microtubule destabilization, which results in lack of motility and profound infertility. This provides evidence for β-defensins being essential for appropriate control of intracellular calcium and regulation of the AR. It is most likely that their loss results in destabilization of the sperm membrane that allows calcium influx and microtubule disruption. This improved understanding of the function of these antimicrobial peptides in mouse leads the way to investigating the role of the human orthologues in sperm function and fertility, and opens the possibility of the development of novel contraceptives with additional antimicrobial action for local use in the female reproductive tract.
COMPETING FINANCIAL INTERESTS
The author declares no competing interest.
ACKNOWLEDGMENTS
I would like to thank my lab. members and all the co-authors of the paper by Zhou et al. 201321 whose hard work made these experiments possible, particularly Yu Zhou, Steve Tardif, Sheila Webb and Fiona Kilanowski. Much of the work from this lecture is based on this paper. Thanks also to the support and enthusiasm of the MRC Human Genetics Unit at the Western General Hospital, Edinburgh where the work21 took place.
REFERENCES
- 1.Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, et al. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA. 2002;99:2129–33. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005;23:5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- 3.Abu BS, Hollox EJ, Armour JA. Allelic recombination between distinct genomic locations generates copy number diversity in human beta-defensins. Proc Natl Acad Sci USA. 2009;106:853–8. doi: 10.1073/pnas.0809073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008;40:23–5. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semple CA, Maxwell A, Gautier P, Kilanowski FM, Eastwood H, et al. The complexity of selection at the major primate beta-defensin locus. BMC Evol Biol. 2005;5:32. doi: 10.1186/1471-2148-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi Y, Nagase T, Makita R, Fukuhara S, Tomita T, et al. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–23. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 7.Zaballos A, Villares R, Albar JP, Martinez A, Marquez G. Identification on mouse chromosome 8 of new beta-defensin genes with regionally specific expression in the male reproductive organ. J Biol Chem. 2004;279:12421–6. doi: 10.1074/jbc.M307697200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou CX, Zhang YL, Xiao L, Zheng M, Leung KM, et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat Cell Biol. 2004;6:458–64. doi: 10.1038/ncb1127. [DOI] [PubMed] [Google Scholar]
- 9.Johnston DS, Turner TT, Finger JN, Owtscharuk TL, Kopf GS, et al. Identification of epididymis-specific transcripts in the mouse and rat by transcriptional profiling. Asian J Androl. 2007;9:522–7. doi: 10.1111/j.1745-7262.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- 10.Diao R, Fok KL, Chen H, Yu MK, Duan Y, et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci Transl Med. 2014;6:249ra108. doi: 10.1126/scitranslmed.3009071. [DOI] [PubMed] [Google Scholar]
- 11.Cao D, Li Y, Yang R, Wang Y, Zhou Y, et al. Lipopolysaccharide-induced epididymitis disrupts epididymal beta-defensin expression and inhibits sperm motility in rats. Biol Reprod. 2010;83:1064–70. doi: 10.1095/biolreprod.109.082180. [DOI] [PubMed] [Google Scholar]
- 12.Tollner TL, Venners SA, Hollox EJ, Yudin AI, Liu X, et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002289. 92ra65. doi: 10.1126/scitranslmed.3002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Diao H, Ni Z, Hu S, Yu H, et al. The epididymis-specific antimicrobial peptide beta-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus) 3. Cell Mol Life Sci. 2011;68:697–708. doi: 10.1007/s00018-010-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yudin AI, Treece CA, Tollner TL, Overstreet JW, Cherr GN. The carbohydrate structure of DEFB126, the major component of the cynomolgus Macaque sperm plasma membrane glycocalyx. J Membr Biol. 2005;207:119–29. doi: 10.1007/s00232-005-0806-z. [DOI] [PubMed] [Google Scholar]
- 15.Morrison GM, Rolfe M, Davidson D, Kilanowski FM, Dorin JR. Genomic organisation and function of members of the murine beta defensin gene family. Pediatr Pulmonol. 2000;20:269. [Google Scholar]
- 16.Morrison GM, Davidson DJ, Kilanowski FM, Borthwick DW, Crook K, et al. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm Genome. 1998;9:453–7. doi: 10.1007/s003359900795. [DOI] [PubMed] [Google Scholar]
- 17.Moser C, Weiner DJ, Lysenko E, Bals R, Weiser JN, et al. Beta-defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun. 2002;70:3068–72. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse Beta defensin 1, defb1, mutant mouse model. Infect Immun. 2002;70:3053–60. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams DJ, Biggs PJ, Cox T, Davies R, van der WL, et al. Mutagenic insertion and chromosome engineering resource (MICER) 15. Nat Genet. 2004;36:867–71. doi: 10.1038/ng1388. [DOI] [PubMed] [Google Scholar]
- 20.Lange UC, Adams DJ, Lee C, Barton S, Schneider R, et al. Normal germ line establishment in mice carrying a deletion of the Ifitm/Fragilis gene family cluster. Mol Cell Biol. 2008;28:4688–96. doi: 10.1128/MCB.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou YS, Webb S, Lettice L, Tardif S, Kilanowski F, et al. Partial deletion of chromosome 8 β-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013;9:e1003826. doi: 10.1371/journal.pgen.1003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semple C, Rolfe M, Dorin J. Duplication and selection in the evolution of primate beta-defensin genes. Genome Biol. 2003;4:R31. doi: 10.1186/gb-2003-4-5-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 24.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011;108:4892–6. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci USA. 2011;108:20008–11. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, et al. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012;26:4198–209. doi: 10.1096/fj.12-205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turunen HT, Sipila P, Krutskikh A, Toivanen J, Mankonen H, et al. Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm-zona pellucida interaction in mice. Biol Reprod. 2012;86:1–8. doi: 10.1095/biolreprod.111.092403. [DOI] [PubMed] [Google Scholar]
- 28.Sato H, Taketomi Y, Isogai Y, Miki Y, Yamamoto K, et al. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J Clin Invest. 2010;120:1400–14. doi: 10.1172/JCI40493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tateno H, Krapf D, Hino T, Sánchez-Cárdenas C, Darszon A, et al. Ca2+ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc Natl Acad Sci USA. 2013;110:18543–8. doi: 10.1073/pnas.1317113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lesich KA, Kelsch CB, Ponichter KL, Dionne BJ, Dang L, et al. The calcium response of mouse sperm flagella: role of calcium ions in the regulation of dynein activity. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.094953. [DOI] [PubMed] [Google Scholar]
- 31.Spelbrink RG, Dilmac N, Allen A, Smith TJ, Shah DM, et al. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004;135:2055–67. doi: 10.1104/pp.104.040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zalazar L, Saez Lancellotti TE, Clementi M, Lombardo C, Lamattina L, et al. SPINK3 modulates mouse sperm physiology through the reduction of nitric oxide level independently of its trypsin inhibitory activity. Reproduction. 2012;143:281–95. doi: 10.1530/REP-11-0107. [DOI] [PubMed] [Google Scholar]


