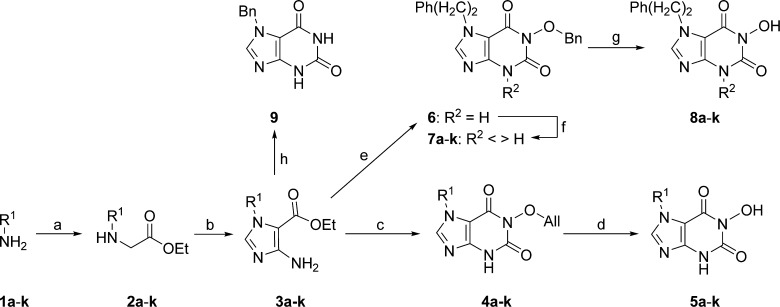
Scheme 1.
Synthesis of 7-substituted 1-hydroxy-3,7-dihydro-1H-purine-2,6-diones. Reagents and conditions: (a) ethyl 2-bromoacetate, CHCl3, rt, 2 h (37–79%); (b) (ethoxymethylene)cyanamide, THF, Δ, 16 h, then KOBut, EtOH, Δ, 2 h (46–80% over 2 steps); (c) CDI, PhMe, Δ, 2 h, then O-allylhydroxylamine, aq NaOH, EtOH, Δ, 2 h (21–61% over 2 steps); (d) Pd(OAc)2, PPh3, HCOOH, EtOH–H2O (8:2), 80 °C, 2 h (23–55%); (e) CDI, PhMe, Δ, 2 h, then O-benzylhydroxylamine, aq NaOH, EtOH, Δ, 2 h (74% over 2 steps); (f) R2X (X = Br or I), K2CO3, DMF, 80 °C, 2–12 h, (67–98%); (g) H2, 10% (w/w) Pd(C), CH2Cl2–MeOH (1:9) (33–53%); (h) urea, 2-methoxyethanol, 190 °C, 24 h, then aq NaOH, Δ, 3 h (33%). For definitions of R1 and R2 refer to Tables 1–3.