Insight from (left to right) Joep Grootjans, Shuhei Hosomi, and Richard Blumberg
Celiac disease (CD) arises from inflammatory T cell and antibody responses to dietary gluten in a genetically susceptible host. CD is notable for a distinct pattern of histological injury to intestinal epithelial cells (IECs) characterized by crypt hyperplasia and atrophy of the villi, which is caused by infiltrating intraepithelial cytotoxic CD8+ T cells (IE-CTLs).
CD is antigen driven and strongly restricted to particular MHC class II alleles such that antigen (gluten) elimination is a recognized therapy. However, emerging data suggest that the “final hit” to disease progression is through downstream noncognate events that are orchestrated by the MHC class II response and may be part of a common spectrum of immune pathways shared with multiple diseases such as inflammatory bowel disease, graft-versus-host disease, and infectious enteritis. Specifically, gluten promotes interleukin-15 (IL-15) expression by distressed IECs via yet to be defined mechanisms. IL-15 in turn recruits additional IE-CTLs to the epithelium and arms these cells by increasing the expression of the activating receptor natural killer group 2 member D (NKG2D). NKG2D ligands, such as MHC class I chain related A and B (MICA/B), are displayed on stressed cells, resulting in their cytolysis by the NKG2D-expressing IE-CTLs, thus setting up a continuous cycle of IEC destruction.
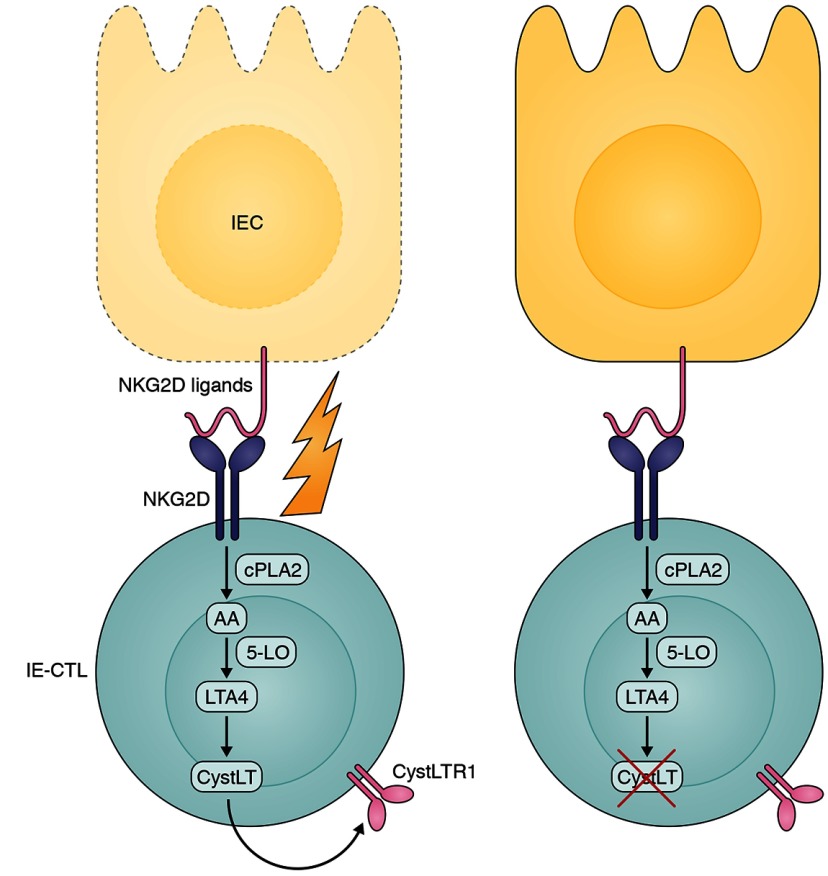
Interaction of NKG2D on IE-CTLs with its ligands expressed on distressed IECs results in a cascade of reactions ultimately leading to increased levels of CystLTs, which is responsible for IEC killing, as observed in patients with CD. Inhibiting CystLTs might decrease IE-CTL cytotoxic capacity and could therefore be an interesting new therapeutic approach in patients with CD.
In this issue, Tang et al. follow up on previous observations showing that NKG2D-mediated cytolysis depends on intracellular signaling via cytosolic phospholipase A2 (cPLA2), which catalyzes release of arachidonic acid (AA) from the membrane. Unexpectedly, they find that the NKG2D-induced cPLA2 and IL-15 signaling pathways converge on the production of leukotrienes, AA metabolites that have been previously linked to allergic disorders including asthma. They find that NKG2D ligation up-regulates an enzymatic cascade associated with 5-lipoxygenase and leukotriene C4 synthase (LTC4S), leading to production of cysteinyl leukotrienes (CystLTs) and the expression of the CystLT receptor on effector cells. Not only do they demonstrate that pharmacological inhibition or siRNA knockdown of enzymes involved in the CystLT pathway significantly impair NKG2D-mediated killing, they show that CystLT up-regulation is demonstrable in patients with active CD but not in healthy controls or in patients on a gluten-free diet.
An FDA-approved CystLT inhibitor, montelukast, is beneficial in patients with asthmatic disease, and the authors show that in vitro pretreatment of IE-CTL lines with montelukast significantly decreases their cytolytic capacity. These experiments unravel an unexpected role for CystLTs in NKG2D-mediated cytolysis and also open up a potential new therapeutic approach for CD that is eminently testable. These exciting results not only underline the benefits of careful and painstaking interrogation of complex signaling pathways but also the opportunities that can be derived from applying clinical insights across widely disparate organ-associated diseases.
References
- Tang, F., et al. 2015. J. Exp. Med. 10.1084/jem.20150303 [DOI] [Google Scholar]