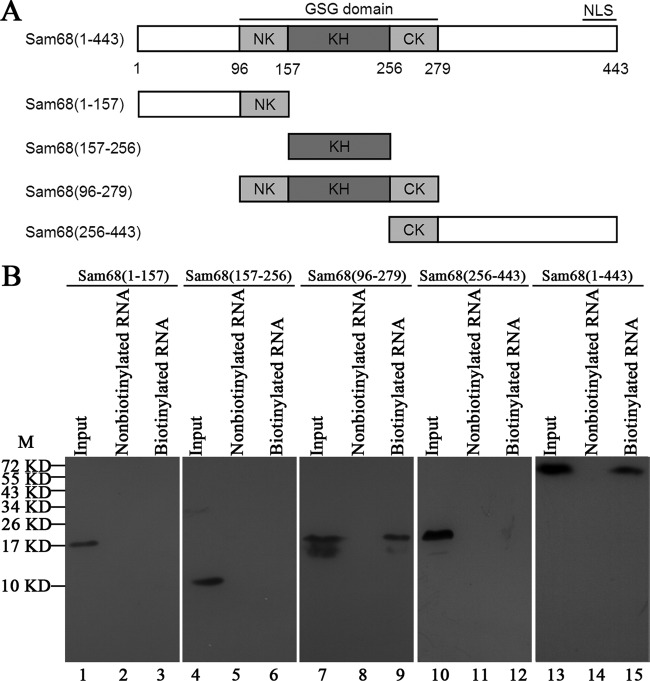
FIG 3.
Regions in the Sam68 protein interacting with EV71 5′UTR. (A) Schematic diagrams of Sam68 and its various truncated mutants. The KH domain is indicated by a dark gray box, and the NK domain and CK domain are indicated by light gray boxes. The other domains are indicated by white boxes. Four truncated forms, Sam68(1-157) (nt 1 to 157 of Sam68), Sam68(157-256), Sam68(96-279), and Sam68(256-443), and full-length Sam68(1-443) were generated and fused with Flag at their N-terminal ends. (B) Expression of full-length Sam68 and its truncated forms in HeLa cells and mapping the interacting regions in Sam68 with EV71 5′UTR. Plasmids that carried full-length Sam68 (lane 13) and various truncated forms of Sam68 (lanes 1, 4, 7, and 10) were transfected into HeLa cells. A Western blot using anti-Flag antibody was employed to examine protein expression. Cell extracts from transfected cells were collected 48 h posttransfection and then incubated either with or without biotinylated EV71 5′UTR. Streptavidin beads were used in the pulldown assay, and the complex was dissolved for Western blot analysis. The amount of “input” was exactly the same (100%) as the total amount of material used in the binding reaction mixture. The positions of molecular mass markers (M) (in kilodaltons) are indicated to the left of the blot.