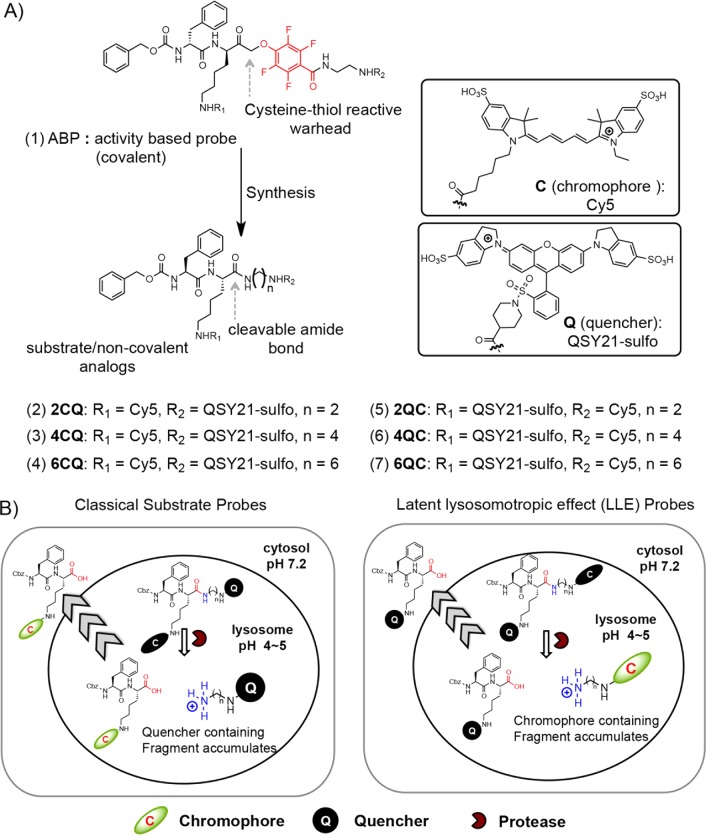
Figure 1.
Design of substrate probes based on an existing quenched fluorescent activity based probe (qABP). (A) Chemical structure of a recently reported broad-spectrum cysteine cathepsin qABP (1) and the design of a set of six substrate analogues by replacement of the phenoxymethyl ketone (PMK) electrophile with a cleavable amide bond. In the nCQ substrates (2–4; where n spacer length = 2, 4, 6), the chromophore (C) is attached to the lysine side chain (R1) and the quenching group (Q) is attached on the C-terminal side of the cleavable amide bond (R2), whereas in the nQC substrates (5–7), the location of each is inverted. (B) Schematic representation of the latent lysosomotropic effect that results in improved retention of the cleaved fluorescent products of probe cleavage.