Abstract
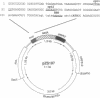
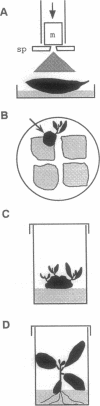
We report here a 100-fold increased frequency of plastid transformation in tobacco by selection for a chimeric aadA gene encoding aminoglycoside 3"-adenylyltransferase, as compared with that obtained with mutant 16S rRNA genes. Expression of aadA confers resistance to spectinomycin and streptomycin. In transforming plasmid pZS197, a chimeric aadA is cloned between rbcL and open reading frame ORF512 plastid gene sequences. Selection was for spectinomycin resistance after biolistic delivery of pZS197 DNA into leaf cells. DNA gel-blot analysis confirmed incorporation of the chimeric aadA gene into the plastid genome by two homologous recombination events via the flanking plastid gene sequences. The chimeric gene became homoplasmic in the recipient cells and is uniformly transmitted to the maternal seed progeny. The ability to transform routinely plastids of land plants opens the way to manipulate the process of photosynthesis and to incorporate novel genes into the plastid genome of crops.
Full text
PDF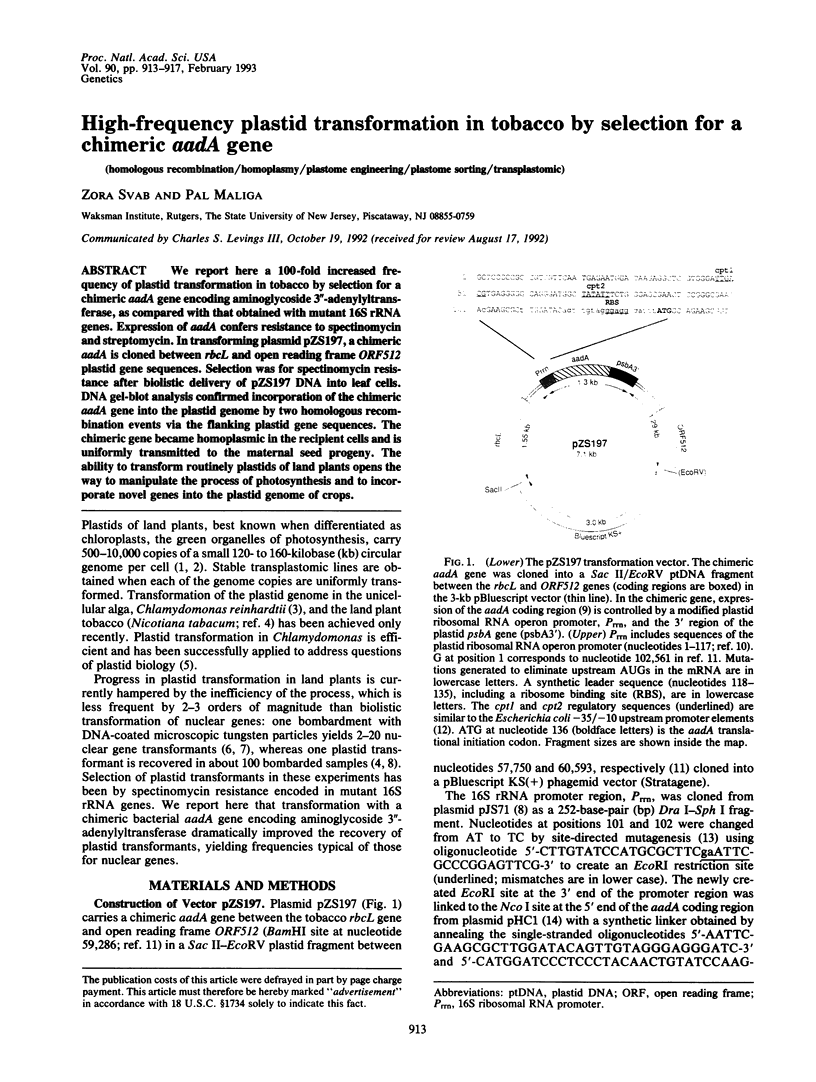
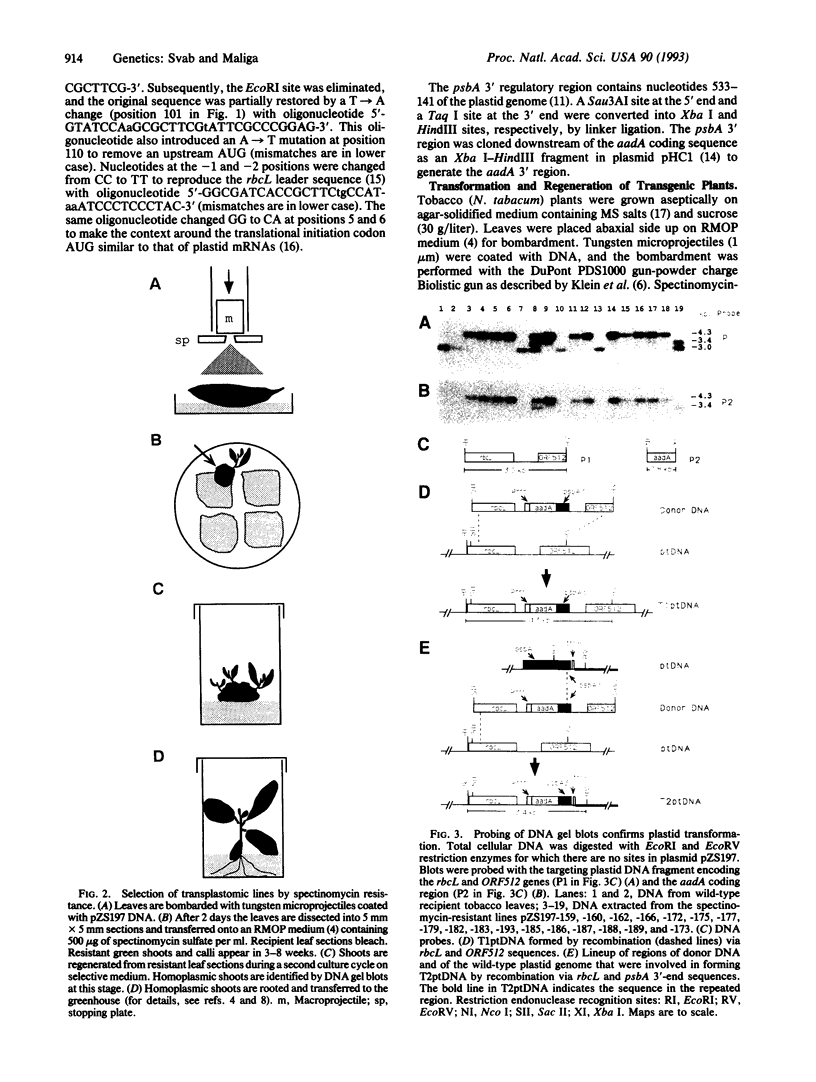
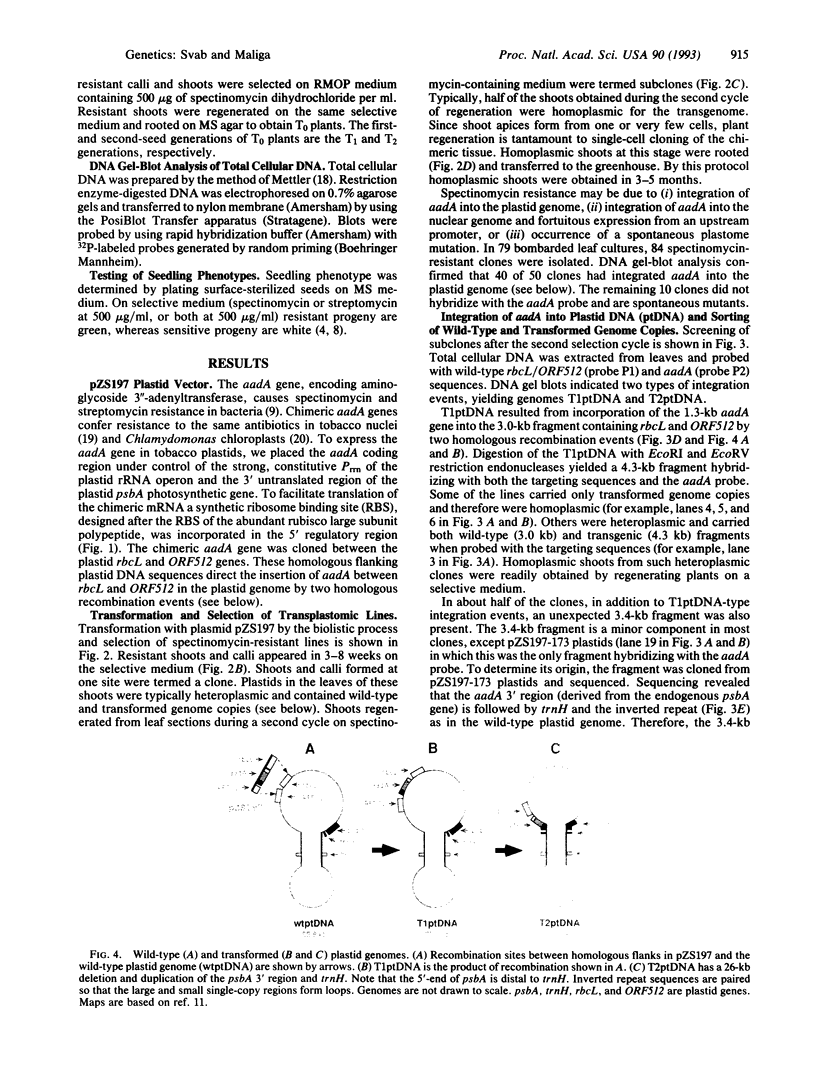


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendich A. J. Why do chloroplasts and mitochondria contain so many copies of their genome? Bioessays. 1987 Jun;6(6):279–282. doi: 10.1002/bies.950060608. [DOI] [PubMed] [Google Scholar]
- Bonham-Smith P. C., Bourque D. P. Translation of chloroplast-encoded mRNA: potential initiation and termination signals. Nucleic Acids Res. 1989 Mar 11;17(5):2057–2080. doi: 10.1093/nar/17.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Carrer H., Staub J. M., Maliga P. Gentamycin resistance in Nicotiana conferred by AAC(3)-I, a narrow substrate specificity acetyltransferase. Plant Mol Biol. 1991 Aug;17(2):301–303. doi: 10.1007/BF00039510. [DOI] [PubMed] [Google Scholar]
- Chinault A. C., Blakesley V. A., Roessler E., Willis D. G., Smith C. A., Cook R. G., Fenwick R. G., Jr Characterization of transferable plasmids from Shigella flexneri 2a that confer resistance to trimethoprim, streptomycin, and sulfonamides. Plasmid. 1986 Mar;15(2):119–131. doi: 10.1016/0147-619x(86)90048-x. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of chlamydomonas. Nucleic Acids Res. 1991 Aug 11;19(15):4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. H., Burkhart B. D., Gillham N. W., Boynton J. E. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989 Oct;123(2):281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Harper E. C., Svab Z., Sanford J. C., Fromm M. E., Maliga P. Stable genetic transformation of intact Nicotiana cells by the particle bombardment process. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8502–8505. doi: 10.1073/pnas.85.22.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield S. P. Chloroplast gene regulation: interaction of the nuclear and chloroplast genomes in the expression of photosynthetic proteins. Curr Opin Cell Biol. 1990 Jun;2(3):509–513. doi: 10.1016/0955-0674(90)90135-2. [DOI] [PubMed] [Google Scholar]
- Melançon P., Lemieux C., Brakier-Gingras L. A mutation in the 530 loop of Escherichia coli 16S ribosomal RNA causes resistance to streptomycin. Nucleic Acids Res. 1988 Oct 25;16(20):9631–9639. doi: 10.1093/nar/16.20.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon P. E., Wagner R., Stutz E. E. coli ribosomes with a C912 to U base change in the 16S rRNA are streptomycin resistant. EMBO J. 1986 Dec 20;5(13):3705–3708. doi: 10.1002/j.1460-2075.1986.tb04703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden C. W., Wolfe K. H., dePamphilis C. W., Palmer J. D. Plastid translation and transcription genes in a non-photosynthetic plant: intact, missing and pseudo genes. EMBO J. 1991 Nov;10(11):3281–3288. doi: 10.1002/j.1460-2075.1991.tb04892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Contrasting modes and tempos of genome evolution in land plant organelles. Trends Genet. 1990 Apr;6(4):115–120. doi: 10.1016/0168-9525(90)90125-p. [DOI] [PubMed] [Google Scholar]
- Shimada H., Sugiura M. Fine structural features of the chloroplast genome: comparison of the sequenced chloroplast genomes. Nucleic Acids Res. 1991 Mar 11;19(5):983–995. doi: 10.1093/nar/19.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub J. M., Maliga P. Long regions of homologous DNA are incorporated into the tobacco plastid genome by transformation. Plant Cell. 1992 Jan;4(1):39–45. doi: 10.1105/tpc.4.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E., Wu B. W., Tewari K. K. In vitro analysis of the pea chloroplast 16S rRNA gene promoter. Mol Cell Biol. 1989 Dec;9(12):5650–5659. doi: 10.1128/mcb.9.12.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Hajdukiewicz P., Maliga P. Stable transformation of plastids in higher plants. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8526–8530. doi: 10.1073/pnas.87.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z., Harper E. C., Jones J. D., Maliga P. Aminoglycoside-3''-adenyltransferase confers resistance to spectinomycin and streptomycin in Nicotiana tabacum. Plant Mol Biol. 1990 Feb;14(2):197–205. doi: 10.1007/BF00018560. [DOI] [PubMed] [Google Scholar]
- Svab Z., Maliga P. Mutation proximal to the tRNA binding region of the Nicotiana plastid 16S rRNA confers resistance to spectinomycin. Mol Gen Genet. 1991 Aug;228(1-2):316–319. doi: 10.1007/BF00282483. [DOI] [PubMed] [Google Scholar]
- Wolfe K. H., Morden C. W., Palmer J. D. Function and evolution of a minimal plastid genome from a nonphotosynthetic parasitic plant. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10648–10652. doi: 10.1073/pnas.89.22.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]