Abstract
Sickle hemoglobin has been expressed in the yeast Saccharomyces cerevisiae after site-directed mutagenesis of a plasmid containing normal human alpha- and beta-globin genes. Cassette mutagenesis of this plasmid was achieved by inserting a DNA fragment containing the beta-globin gene in the replicative form of M13mp18 to make a point mutation and then reconstituting the original plasmid containing the mutated beta-globin gene. Pure recombinant hemoglobin S was shown to be identical to natural sickle hemoglobin in its ultraviolet and visible absorption bands and by gel electrophoresis, isoelectric focusing, amino acid analysis, mass spectrometry, partial N-terminal sequencing, and functional properties (P50, cooperativity, and response to 2,3-bisphosphoglycerate). In yeast and in mammalian cells, cotranslational processing yields the same N-terminal valine residues of hemoglobin alpha- and beta-chains, but in bacterial expression systems the N terminus is extended by an additional amino acid because the initiator methionine residue is retained. Since the N-terminal valine residues of both chains of hemoglobin S participate in important physiological functions, such as oxygen affinity, interaction with anions, and the Bohr coefficient, the yeast expression system is preferable to the bacterial system for recombinant DNA studies. Hence, mutagenesis employing this expression system should permit definitive assignments of the role of any amino acid side chain in hemoglobin S aggregation and could suggest additional approaches to therapeutic intervention. The engineering of this system for the synthesis of sickle hemoglobin and its purification to homogeneity in a single column procedure are described.
Full text
PDF
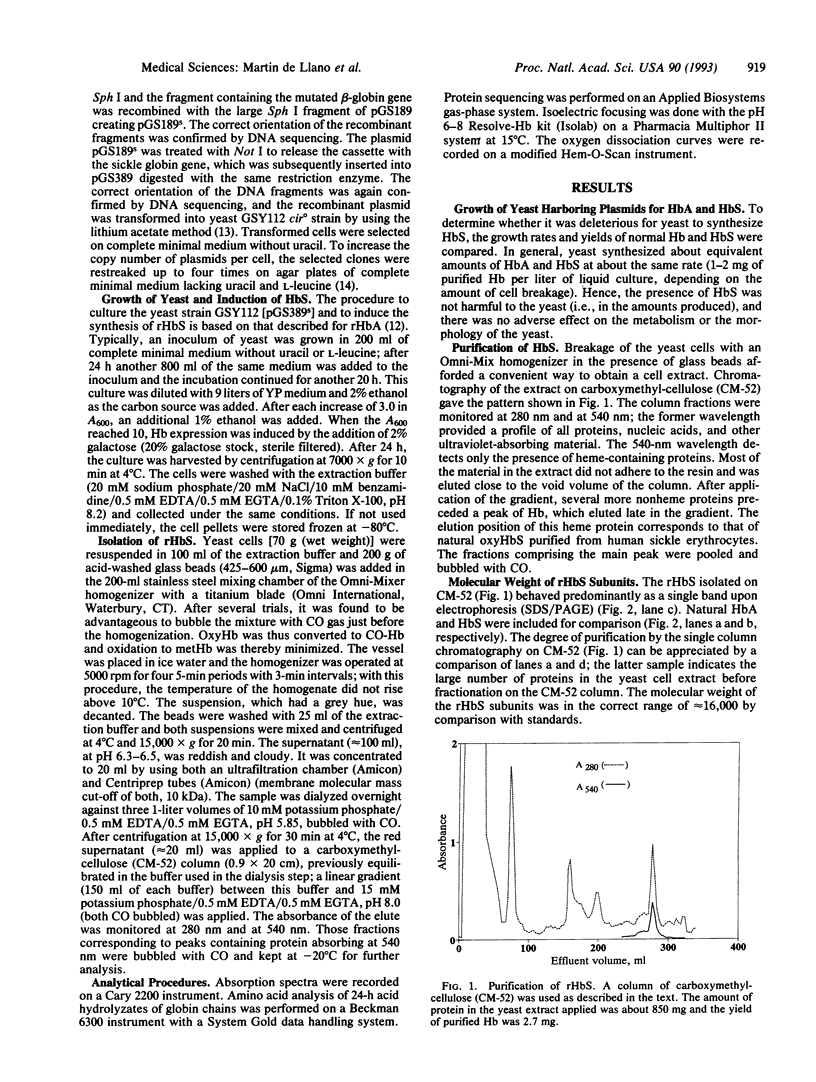

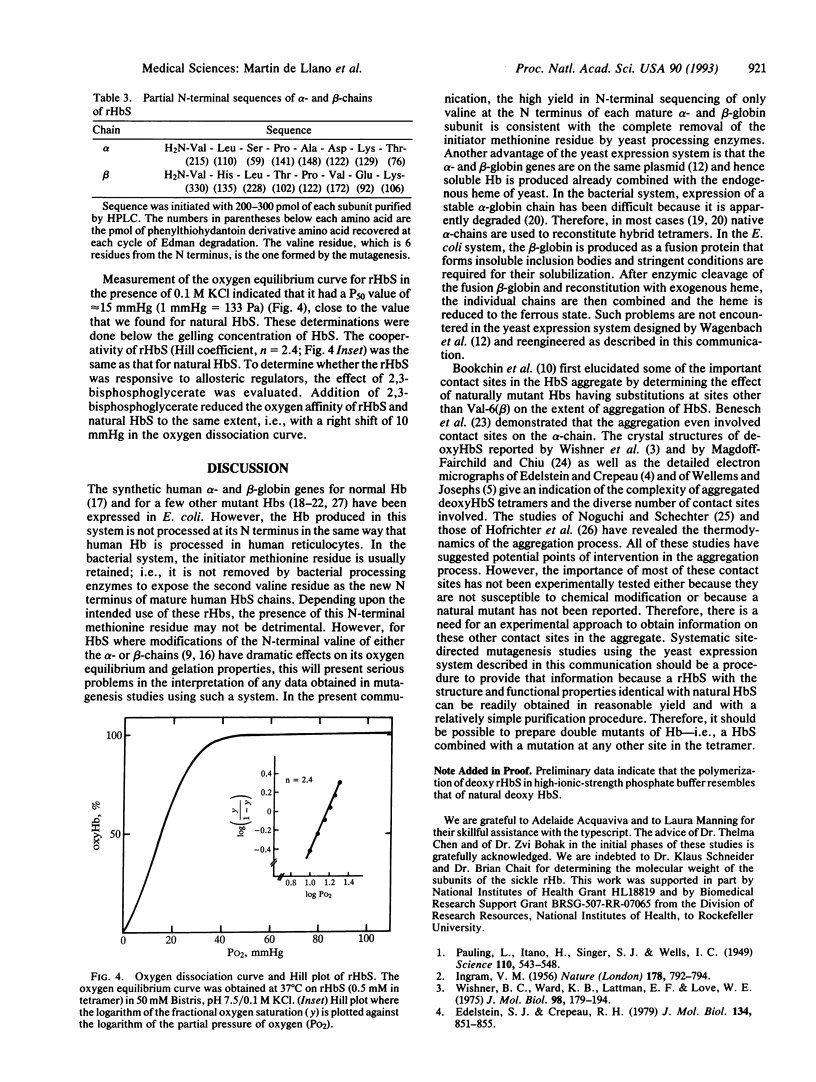

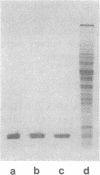
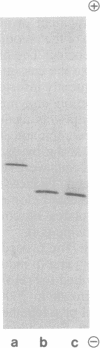
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Rappaport E., Eck H. S., Konitzer P., Kim J., Surrey S. Polymerization and solubility of recombinant hemoglobins alpha 2 beta 2 (6Val) (Hb S) and alpha 2 beta 2(6Leu) (Hb Leu). Hemoglobin. 1991;15(5):417–430. doi: 10.3109/03630269108998861. [DOI] [PubMed] [Google Scholar]
- Armstrong K. A., Som T., Volkert F. C., Rose A., Broach J. R. Propagation and expression of genes in yeast using 2-micron circle vectors. Biotechnology. 1989;13:165–192. [PubMed] [Google Scholar]
- Baudin-Chich V., Pagnier J., Marden M., Bohn B., Lacaze N., Kister J., Schaad O., Edelstein S. J., Poyart C. Enhanced polymerization of recombinant human deoxyhemoglobin beta 6 Glu----Ile. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1845–1849. doi: 10.1073/pnas.87.5.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesch R. E., Kwong S., Edalji R., Benesch R. alpha Chain mutations with opposite effects on the gelation of hemoglobin S. J Biol Chem. 1979 Sep 10;254(17):8169–8172. [PubMed] [Google Scholar]
- Bihoreau M. T., Baudin V., Marden M., Lacaze N., Bohn B., Kister J., Schaad O., Dumoulin A., Edelstein S. J., Poyart C. Steric and hydrophobic determinants of the solubilities of recombinant sickle cell hemoglobins. Protein Sci. 1992 Jan;1(1):145–150. doi: 10.1002/pro.5560010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M. Structure and properties of hemoglobin C-Harlem, a human hemoglobin variant with amino acid substitutions in 2 residues of the beta-polypeptide chain. J Biol Chem. 1967 Jan 25;242(2):248–255. [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (third of three parts). N Engl J Med. 1978 Oct 19;299(16):863–870. doi: 10.1056/NEJM197810192991605. [DOI] [PubMed] [Google Scholar]
- Doyle M. L., Lew G., De Young A., Kwiatkowski L., Wierzba A., Noble R. W., Ackers G. K. Functional properties of human hemoglobins synthesized from recombinant mutant beta-globins. Biochemistry. 1992 Sep 15;31(36):8629–8639. doi: 10.1021/bi00151a033. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J., Crepeau R. H. Oblique alignment of hemoglobin S fibers in sickled cells. J Mol Biol. 1979 Nov 15;134(4):851–855. doi: 10.1016/0022-2836(79)90491-1. [DOI] [PubMed] [Google Scholar]
- Hernan R. A., Hui H. L., Andracki M. E., Noble R. W., Sligar S. G., Walder J. A., Walder R. Y. Human hemoglobin expression in Escherichia coli: importance of optimal codon usage. Biochemistry. 1992 Sep 15;31(36):8619–8628. doi: 10.1021/bi00151a032. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh J. S., Rogers P. H., Arnone A. High-resolution X-ray study of deoxy recombinant human hemoglobins synthesized from beta-globins having mutated amino termini. Biochemistry. 1992 Sep 15;31(36):8640–8647. doi: 10.1021/bi00151a034. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdoff-Fairchild B., Chiu C. C. X-ray diffraction studies of fibers and crystals of deoxygenated sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):223–226. doi: 10.1073/pnas.76.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J. M. Covalent inhibitors of the gelation of sickle cell hemoglobin and their effects on function. Adv Enzymol Relat Areas Mol Biol. 1991;64:55–91. doi: 10.1002/9780470123102.ch2. [DOI] [PubMed] [Google Scholar]
- Manning J. M. Preparation of hemoglobin carbamylated at specific NH2-terminal residues. Methods Enzymol. 1981;76:159–167. doi: 10.1016/0076-6879(81)76124-x. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- PAULING L., ITANO H. A. Sickle cell anemia a molecular disease. Science. 1949 Nov 25;110(2865):543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- Wagenbach M., O'Rourke K., Vitez L., Wieczorek A., Hoffman S., Durfee S., Tedesco J., Stetler G. Synthesis of wild type and mutant human hemoglobins in Saccharomyces cerevisiae. Biotechnology (N Y) 1991 Jan;9(1):57–61. doi: 10.1038/nbt0191-57. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Josephs R. Crystallization of deoxyhemoglobin S by fiber alignment and fusion. J Mol Biol. 1979 Dec 15;135(3):651–674. doi: 10.1016/0022-2836(79)90170-0. [DOI] [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]