Summary
Background
Gastrostomy feeding is commonly used to support patients with amyotrophic lateral sclerosis who develop severe dysphagia. Although recommended by both the American Academy of Neurology and the European Federation of Neurological Societies, currently little evidence indicates the optimum method and timing for gastrostomy insertion. We aimed to compare gastrostomy insertion approaches in terms of safety and clinical outcomes.
Methods
In this large, longitudinal, prospective cohort study (ProGas), we enrolled patients with a diagnosis of definite, probable, laboratory supported, or possible amyotrophic lateral sclerosis who had agreed with their treating clinicians to undergo gastrostomy at 24 motor neuron disease care centres or clinics in the UK. The primary outcome was 30-day mortality after gastrostomy. This study was registered on the UK Clinical Research Network database, identification number 9923.
Findings
Between Nov 2, 2010, and Jan 31, 2014, 345 patients were recruited of whom 330 had gastrostomy. 163 (49%) patients underwent percutaneous endoscopic gastrostomy, 121 (37%) underwent radiologically inserted gastrostomy, 43 (13%) underwent per-oral image-guided gastrostomy, and three (1%) underwent surgical gastrostomy. 12 patients (4%, 95% CI 2·1–6·2) died within the first 30 days after gastrostomy: five (3%) of 163 after percutaneous endoscopic gastrostomy, four (3%) of 121 after radiologically inserted gastrostomy, and three (7%) of 43 after per-oral image-guided gastrostomy (p=0·46). Including repeat attempts in 14 patients, 21 (6%) of 344 gastrostomy procedures could not be completed: 11 (6%) of 171 percutaneous endoscopic gastrostomies, seven (6%) of 121 radiologically inserted gastrostomies, and three (6%) of 45 per-oral image-guided gastrostomies (p=0·947).
Interpretation
The three methods of gastrostomy seemed to be as safe as each other in relation to survival and procedural complications. In the absence of data from randomised trials, our findings could inform clinicians and patients in reaching decisions about gastrostomy and will stimulate further research into the nutritional management in patients with amyotrophic lateral sclerosis.
Funding
Motor Neurone Disease Association of Great Britain and Northern Ireland (MNDA) and the Sheffield Institute for Translational Neuroscience (SITraN).
Introduction
Amyotrophic lateral sclerosis is a neurodegenerative illness causing progressive weakness and wasting of muscles controlling movement, breathing, and swallowing.1 Dysphagia is a common problem in patients with amyotrophic lateral sclerosis and causes difficulties in maintaining a safe and adequate oral intake of nutrition and fluids.2 Patients with severe dysphagia often experience weight loss, choking, and coughing on attempting to swallow, episodes of aspiration, and prolonged and effortful mealtimes.3–6
Gastrostomy feeding is recommended to provide long-term nutritional support for patients with amyotrophic lateral sclerosis with severe dysphagia.7 Three main methods of gastrostomy insertion are currently used in patients with amyotrophic lateral sclerosis: percutaneous endoscopic gastrostomy, radiologically inserted gastrostomy, and per-oral image-guided gastrostomy.8 However, with little evidence available,9,10 current practice in relation to choice of method and timing of gastrostomy insertion is largely based on consensus and expert opinion.8 Gastrostomy could be beneficial for the survival, quality of life, and nutritional outcome of patients with this disease, but there is a paucity of high-quality evidence relating to these aspects of the intervention.9,11–13
In response to the paucity of evidence and calls by organisations such as the American Academy of Neurology and the European Federation of Neurological Societies for more evidence to guide clinicians and optimise standards of care,7,14 we aimed to identify the optimum gastrostomy timing and insertion method in terms of safety and clinical outcomes.
Methods
Study design and participants
In this large, multicentre, longitudinal, prospective cohort study (ProGas), we enrolled patients with a diagnosis of definite, probable, laboratory supported, or possible amyotrophic lateral sclerosis (as defined by the El Escorial criteria),15 who had agreed with their clinicians to undergo gastrostomy at one of 24 motor neuron disease care centres or clinics in the UK (21 in England, two in Scotland, and one in Northern Ireland). Patients who had been diagnosed with a disorder characterised by cognitive impairment, such as frontotemporal dementia, were excluded. Patients were approached and invited to take part in the study by a member of the research team when a decision had been made to refer the patient for a gastrostomy insertion. Ethical approval was granted by the National Health Service NRES Leeds (Central) Research Ethics Committee and applied to all participating care centres or clinics. Informal carers, such as family members, of patients who had accepted to take part in the study were also invited to participate. All participants who agreed to take part in the study provided written informed consent before data collection.
Research in context.
Evidence before this study
We searched PubMed, Embase, The Cochrane Library, and ISI Web of Knowledge for reports published before July 1, 2010, combined with citation searching and reference chaining, using the keywords: “motor neuron* disease” or “MND”, “amyotrophic lateral sclerosis” or “ALS”, “gastrostomy”, “percutaneous endoscopic gastrostomy” or “PEG”, “radiologically-inserted gastrostomy” or “RIG”, “per-oral image-guided gastrostomy” or “PIG”, “timing”, “mortality”, “safety”, “nutritional outcome”, “benefits”, and “quality of life”. We identified several studies reporting mortality data after gastrostomy insertion in patients with amyotrophic lateral sclerosis, but only a handful directly compared survival time or 30-day post-procedure mortality after different methods of gastrostomy. In a meta-analysis of the data of the four studies that allowed within-study comparisons of percutaneous endoscopic gastrostomy versus radiologically inserted gastrostomy or per-oral image-guided gastrostomy, the difference in 30-day mortality was increased by 2·1% for percutaneous endoscopic gastrostomy compared with the other insertion methods. However, the results of the meta-analysis did not provide robust evidence to indicate which method is safer because of an absence of within-study comparisons, differences between populations, small sample sizes, and low event rates. The urgent need for prospective clinical trials in relation to the optimum method and timing for gastrostomy insertion, as well as the nutritional outcome for the patients, was highlighted in a Cochrane review on enteral tube feeding for amyotrophic lateral sclerosis and echoed in calls for more robust evidence by multiple organisations, such as the American Academy of Neurology and the European Federation of Neurological Societies.
Added value of this study
To our knowledge, ProGas is the first large, multicentre, longitudinal, cohort study to assess and compare the different methods of gastrostomy and explore the issue of optimal timing for gastrostomy insertion in patients with amyotrophic lateral sclerosis.
Implications of all the available evidence
In the absence of data from randomised trials, our findings might help neurologists, patients with amyotrophic lateral sclerosis, and the carers of patients with amyotrophic sclerosis to make decisions about the timing and method of gastrostomy. The next steps in building the evidence base must be to understand further the nutritional requirements of patients with amyotrophic lateral sclerosis, particularly the quantity and quality of nutritional support that patients receive after gastrostomy, and to explore the factors that can lead to continuing weight loss after the procedure.
Procedures
Adhering to the study protocol and the National Institute for Health Research guidelines for good clinical practice, data collection was carried out by experienced members of the local research teams. Data were collected at four timepoints: at the time of recruitment (baseline), at the end of the gastrostomy procedure, at 3 months after gastrostomy, and at 12 months after gastrostomy. At baseline, we collected the following information: demographic characteristics; clinician opinion on indication, timing, potential benefits, and preferred type of gastrostomy; patient's influence on the timing of gastrostomy; measures of respiratory function; and indices of disease progression. At baseline, 3 months, and 12 months we collected the following information: demographic characteristics, weight, height, and score on the revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R).16 Data related to the operation itself such as gastrostomy equipment, type of gastrostomy tube used, procedure length, and details of any complications were collected at the end of the gastrostomy procedure. At baseline and 3 months after gastrostomy insertion, patients who gave consent were asked to complete a questionnaire assessing quality of life (MQOL)17 and a questionnaire assessing the strain of caregiving activities was completed by consenting informal carers (MCSI).18
Outcomes
The primary outcome of the study was 30-day mortality after gastrostomy.8 The secondary outcomes were perigastrostomy and post-gastrostomy complication rate (defined as complications that occurred during the gastrostomy procedure and those that occurred at any timepoint in the first 3 months after completion of the gastrostomy insertion procedure, respectively), median survival time from gastrostomy placement, nutritional status change, self-perceived quality of life changes after gastrostomy, and changes in carer strain after gastrostomy.
Statistical analysis
Assuming a 30-day mortality rate of 5%, based on a meta-analysis of the available literature,8 to estimate mortality within greater or less than 2·5% (ie, 95% CI 2·5–7·5) would require 30-day mortality data for a minimum of 300 patients with amyotrophic lateral sclerosis. Current European Federation of Neurological Societies guidelines recommend gastrostomy after weight loss of at least 10% from premorbid weight.14 This threshold was used in our study to classify patients into weight loss subgroups for subsequent analyses. Continuity corrected χ2 tests were done to determine the difference in the 30-day mortality and the complication rates after gastrostomy in patients who underwent percutaneous endoscopic gastrostomy, radiologically inserted gastrostomy, or per-oral image-guided gastrostomy. Kaplan-Meier survival curves were used to determine the median survival time from placement and disease onset for the treatment groups. Cox proportional hazards regression analysis was done to determine predictors of survival from the time of gastrostomy insertion and from the time of disease onset (to take into account variables that have an effect on survival over the whole course of the disease). Our rationale for inclusion of covariates in the Cox regression analysis was based on well known factors that might affect survival in patients with amyotrophic lateral sclerosis as reported previously, and on our clinical judgment of other probable factors that might affect survival post gastrostomy. Continuity corrected χ2 tests were used to determine changes in nutritional status in the patients who underwent percutaneous endoscopic gastrostomy, radiologically inserted gastrostomy, or per-oral image-guided gastrostomy. Cox proportional hazards regression analysis was also done to examine the effect of nutritional status at 3 months post gastrostomy on subsequent survival. Cronbach's α coefficients were determined for the quality of life and strain measures used in the study to assess their internal consistency. A paired samples t test was used to determine differences in the self-perceived quality of life of the patients and the strain of caregiving activities of carers. We obtained complete mortality data for all patients who underwent gastrostomy. Initially, complete case analysis was done—ie, patients who had one or more missing values in the variables being analysed were omitted from the analysis pairwise. To compensate for missing data, post-hoc multiple imputation was done for the covariates of interest in our multiple regression analyses. Because ProGas was not a randomised controlled trial, we addressed the issue of treatment indication bias by undertaking a post-hoc propensity score analysis (appendix p 3).
Data were managed and analysed with SPSS Statistics for Windows version 21.0.
Role of the funding source
This study was supported jointly by the Motor Neurone Disease Association of England, Wales, and Northern Ireland and the Sheffield Institute for Translational Neuroscience. Both funding bodies were consulted regarding the study design, and the decision to submit the report for publication fulfils their requirement for dissemination of the findings. However, the funding sources were not involved in data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all of the data and CJM had final responsibility for the decision to submit the report for publication.
Results
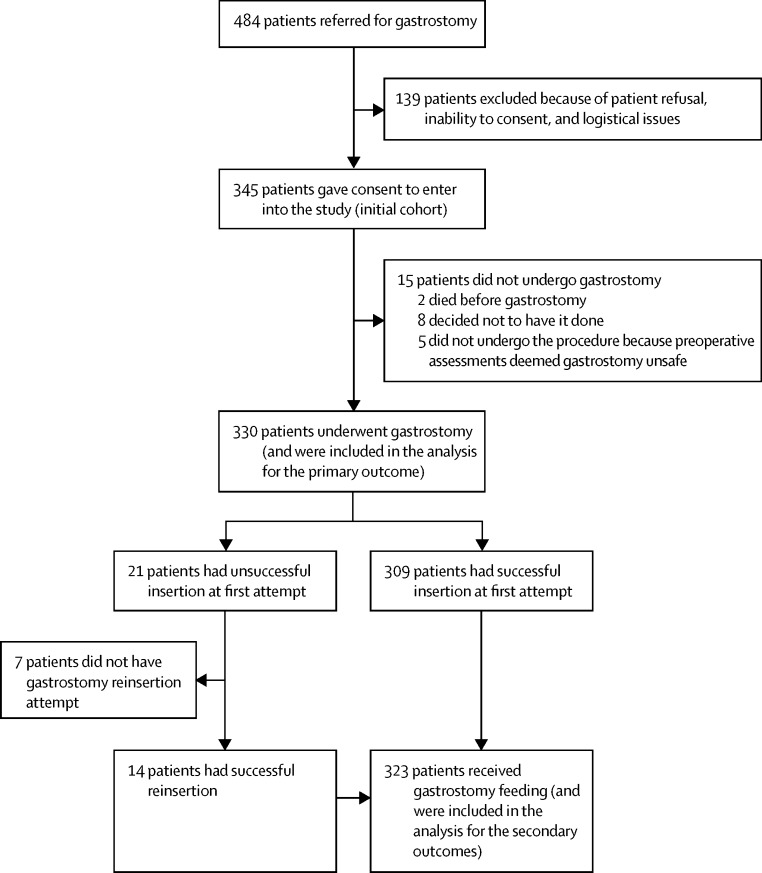
Between Nov 2, 2010, and Jan 31, 2014, 330 patients underwent gastrostomy and were included in the analysis for the primary outcome (figure 1). Table 1 shows their baseline characteristics. 163 (49%) patients underwent percutaneous endoscopic gastrostomy, 121 (37%) underwent radiologically inserted gastrostomy, 43 (13%) underwent per-oral image-guided gastrostomy, and three (1%) underwent surgical gastrostomy. Table 2 summarises the differences across the three gastrostomy groups. Data for criteria used for gastrostomy method selection, indication, and predicted benefits on influence of patients on timing of gastrostomy, and on types and sizes of gastrostomy tubes are available on appendix p 1.
Figure 1.
Study profile
Table 1.
Baseline demographic and clinical characteristics
| Baseline value in all patients (n=330) | ||
|---|---|---|
| Age (years) | 64·4 (11·7), n=315 | |
| Sex | ||
| Women | 150/330 (45%) | |
| Men | 180/330 (55%) | |
| Forced vital capacity (%) | 62% (22·6), n=258 | |
| % Weight loss from diagnosis to baseline (kg) | 8·6 (9·8), n=252 | |
| ALSFRS-R score | 28 (8·5), n=307 | |
| Body-mass index (kg/m2) | 23·3 (4·4), n=274 | |
| Monthly ALSFRS-R decline | 2·2% (1·7%), n=290 | |
| Disease duration from diagnosis (months) | 16·7 (5·8–14·9), n=309 | |
| Non-invasive ventilation routine users | 81/323 (25%) | |
| Site of disease onset | ||
| Limb | 152/324 (47%) | |
| Bulbar | 165/324 (51%) | |
| Both limb and bulbar | 6/324 (2%) | |
| Respiratory | 1/324 (0·3%) | |
Data are mean (SD), median (IQR), or n/N (%). ALSFRS-R=amyotrophic lateral sclerosis functional rating scale revised.
Table 2.
Baseline differences of patients who underwent PEG, RIG, or PIG
| PEG | RIG | PIG | Statistic (n) | p value | ||
|---|---|---|---|---|---|---|
| Age (years) | 64·2 (11·7), n=157 | 63·6 (9·8), n=114 | 67·2 (12·6), n=41 | F (312) | 0·200 | |
| Sex | χ2 (327) | 0·683 | ||||
| Women | 73/163 (45%) | 59/121 (49%) | 18/43 (42%) | |||
| Men | 90/163 (55%) | 62/121 (51%) | 25/43 (58%) | |||
| FVC (%) | 65·4 (22·2), n=136 | 59 (23·1), n=87 | 52 (19·7), n=33 | F (256) | 0·004 | |
| % Weight loss from diagnosis to baseline (kg) | 7·1 (8·5), n=117 | 8·7 (9·9), n=98 | 13 (12·3), n=35 | F (250) | 0·008 | |
| ALSFRS-R score | 29·1 (8·2), n=152 | 27·7 (8·8), n=114 | 24·7 (7·9), n=39 | F (305) | 0·014 | |
| Body-mass index (kg/m2) | 23·7 (4), n=135 | 23·4 (5·1), n=102 | 21·8 (3), n=34 | F (271) | 0·091 | |
| Monthly ALSFRS-R decline | 2·1% (1·5), n=144 | 2·4% (2·1), n=105 | 2·1% (1·2), n=39 | F (288) | 0·302 | |
| NIV routine users | 29/162 (18%) | 23/118 (19%) | 28/42 (67%) | χ2 (322) | 0·001 | |
| Site of disease onset | χ2 (321) | 0·369 | ||||
| Limb | 74/161 (46%) | 54/117 (46%) | 23/43 (53%) | |||
| Bulbar | 86/161 (53%) | 59/117 (50%) | 18/43 (42%) | |||
| Both limb and bulbar | 1/161 (1%) | 3/117 (3%) | 2/43 (5%) | |||
| Respiratory | 0/161 | 1/117 (1%) | 0/43 | |||
Data are mean (SD) or n/N (%). PEG=percutaneous endoscopic gastrostomy. RIG=radiologically inserted gastrostomy. PIG=per-oral image-guided gastrostomy. F=one-way ANOVA F test. FVC=forced vital capacity. ALSFRS-R=amyotrophic lateral sclerosis functional rating scale revised. NIV=non-invasive ventilation.
The study was funded for 38 months and stopped on Jan 31, 2013, at which point all patients had undergone data collection for the primary outcome. Nine patients did not undergo formal 3-month assessments and 93 patients did not undergo 12-month assessments.
12 (4%, 95% CI 2–6) of 330 patients died within the first 30 days after gastrostomy: five (3%, 1–7) of 163 after percutaneous endoscopic gastrostomy, four (3%, 1–8) of 121 after radiologically inserted gastrostomy, and three (7%, 2–19) of 43 after per-oral image-guided gastrostomy (p=0·46). We did not find evidence of a difference in 30-day mortality between the procedures after adjustment for case mix variables (age at onset, weight loss, functional decline rate, forced vital capacity, and site of onset) and treatment centre (appendix p 2).
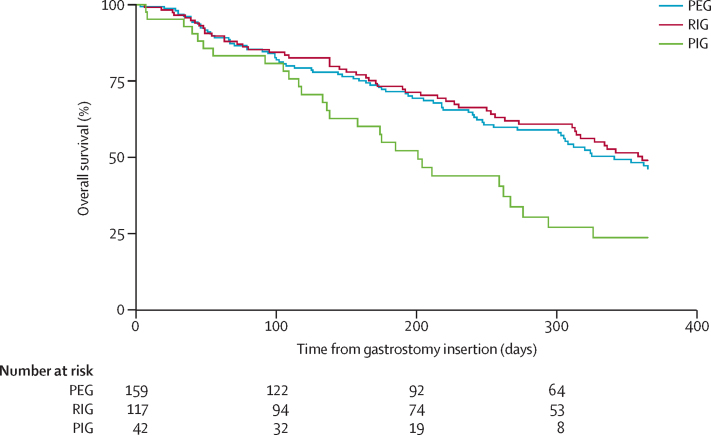
Overall median survival after gastrostomy was 325 days (95% CI 289–361). Median survival time after percutaneous endoscopic gastrostomy was 341 days (25th IQR inderteminate–164), after radiologically inserted gastrostomy was 361 days (25th IQR inderteminate–171), and after per-oral image-guided gastrostomy was 201 days (326–116; figure 2). We noted some evidence of a difference in survival times (log-rank χ2 1·4 on 2 df, p=0·03) between the three gastrostomy insertion methods before any adjustment for case mix variables (age at onset, weight loss, functional decline rate, forced vital capacity, and site of onset) and treatment centre. However, after adjustment for treatment centre and case mix variables, we did not note any evidence of a difference in survival times between the three gastrostomy insertion methods (appendix p 2).
Figure 2.
Survival functions for patients who underwent PEG, RIG, or PIG
Subsequent Cox proportional hazards analysis suggested that the method of gastrostomy insertion was not significantly associated with survival. PEG=percutaneous endoscopic gastrostomy. RIG=radiologically inserted gastrostomy. PIG=per-oral image-guided gastrostomy.
Irrespective of method of gastrostomy, among the 12 patients who died within the first 30 days following the procedure, one patient (8%) had lost up to 10% of their bodyweight compared with that at diagnosis (2·6% loss), eight patients (67%) had lost more than 10% of their weight (mean 17·1% [SD 5·6]), one patient (8%) had gained weight (3·1% gain; χ2, n=252, p=0·031), and for two patients (17%) weight data were missing. Binary logistic regression analysis showed that the odds for 30-day mortality were 10·7 times higher (95% CI 1·3–87·0; p=0·027) for patients who had lost more than 10% of their weight from diagnosis compared with those who had lost 10% or less of weight.
Cox proportional hazards regression was done to ascertain the effect of gastrostomy method on survival from the time of gastrostomy insertion, with adjustment for covariates that might also affect survival. Variables that were inserted into the regression model were gastrostomy insertion method (percutaneous endoscopic gastrostomy, radiologically inserted gastrostomy, and per-oral image-guided gastrostomy subgroups), forced vital capacity at the time of gastrostomy insertion, percentage of weight difference at gastrostomy compared with diagnosis weight, and three additional well established predictors of survival in patients with amyotrophic lateral sclerosis:19 age at the onset of amyotrophic lateral sclerosis, site of amyotrophic lateral sclerosis symptom onset (bulbar and limb subgroups), and monthly rate of decline of the revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R). The results showed that the hazard of death after gastrostomy insertion was significantly affected by two main factors: the age at onset of amyotrophic lateral sclerosis (hazard ratio [HR] 1·032, 95% CI 1·007–1·059; p=0·013) and the percentage of weight difference at gastrostomy compared with weight at diagnosis (HR 0·956, 0·930–0·983; p=0·001). The hazard of death was not affected by the gastrostomy insertion method. Figure 2 shows the survival functions for the subgroups of patients who underwent percutaneous endoscopic gastrostomy, radiologically inserted gastrostomy, or per-oral image-guided gastrostomy.
A Cox proportional hazards regression model including the same variables showed that the hazard of death from the time of amyotrophic lateral sclerosis onset was significantly affected by the age at onset (HR 1·045 [95% CI 1·015–1·075]; p=0·003), the ALSFRS-R monthly decline rate (1·768 [1·541–2·028]; p=0·001), and the site of amyotrophic lateral sclerosis symptom onset (bulbar compared with limb subgroups, HR 2·082 [1·204–3·601]; p=0·009).
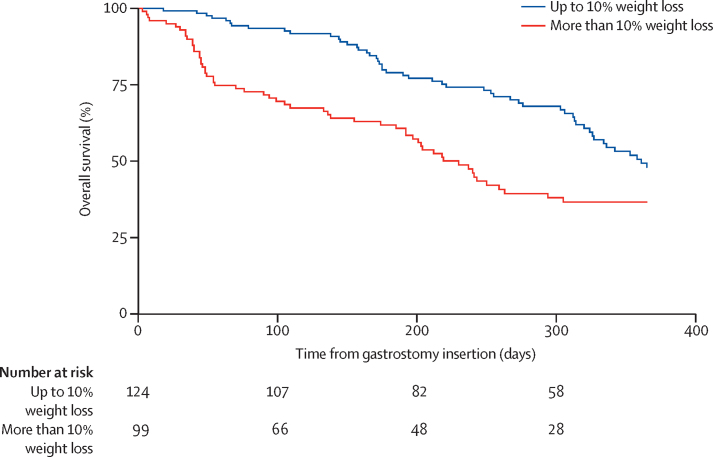
To further explore the effect of weight loss on survival after insertion, we did a Cox proportional hazards analysis with adjustment for covariates that might also affect survival. The regression model included as variables weight at the time of gastrostomy compared with weight at diagnosis (<10% weight loss and >10% weight loss subgroups), forced vital capacity at the time of gastrostomy insertion, age at the onset of amyotrophic lateral sclerosis, site of amyotrophic lateral sclerosis symptom onset (bulbar and limb subgroups), and ALSFRS-R monthly decline rate. Hazard of death after gastrostomy insertion was significantly affected by the age at onset (HR 1·035 [95% CI 1·008–1·063]; p=0·011) and the percentage of weight loss from diagnosis to gastrostomy (>10% weight loss subgroup compared with the <10% weight loss subgroup, 2·514 [1·490–4·243]; p=0·001). The median survival after gastrostomy for patients who had lost 10% or less of weight from diagnosis was 12 months (95% CI was indeterminate because survival was greater than 50% at the last timepoint in this subgroup) and for those who had lost more than 10% of their weight from diagnosis was 7·7 months (n=223, 95% CI 6·5–8·9; log-rank test p=0·001). Figure 3 shows the survival functions for the different subgroups of patients in terms of weight loss at gastrostomy compared with weight at the time of diagnosis.
Figure 3.
Survival functions according to weight loss
Periprocedural complications did not differ significantly across the three gastrostomy insertion methods, apart from the higher perioperational distress experienced by percutaneous endoscopic gastrostomy patients (table 3). Table 4 summarises complications in the first 3 months after gastrostomy. Patients who received balloon-retention tubes (radiologically inserted gastrostomy) had a significantly higher rate of tube-related complications than did those who received bumper-retention tubes, including displacement (20 [31%] of 96 patients vs one [1%] of 154 patients; p=0·001), leakage (21 [22%] vs 16 [10%]; p=0·011), replacement (29 [30%] vs four [3%]; p=0·001), and repeated gastrostomy (14/96 [15%] vs one [1%]; 0·001; appendix p 1); percutaneous endoscopic gastrostomy and per-oral image-guided gastrostomy odds ratio data are given in appendix p 1).
Table 3.
Periprocedural complications
| PEG | RIG | PIG | Total | p value | |
|---|---|---|---|---|---|
| Overall complication rate | 41/169 (24%) | 20/121 (17%) | 8/42 (19%) | 69/332 (21%) | 0·266 |
| Difficult procedure | 26/168 (16%) | 13/119 (11%) | 7/42 (17%) | 46/329 (14%) | 0·475 |
| Failed attempt | 11/171 (6%) | 7/125 (6%) | 3/45 (7%) | 21/341 (6%) | 0·947 |
| O2desaturation | 6/166 (4%) | 2/117 (2%) | 3/42 (7%) | 11/325 (3%) | 0·241 |
| Patient distress | 26/166 (16%) | 4/117 (3%) | 2/42 (5%) | 32/325 (10%) | 0·002 |
| Respiratory arrest | 0/166 | 0/117 | 0/42 | 0/325 | NA |
| Laryngeal spasm | 2/166 (1%) | 1/117 (1%) | 0/42 | 3/325 (1%) | 0·763 |
| Haemorrhage | 0/166 | 3/117 (3%) | 0/42 | 3/325 (1%) | 0·068 |
Numbers are patients who experienced each event (n)/total patients in each group (N). The periprocedural period is the time during the gastrostomy procedure. PEG=percutaneous endoscopic gastrostomy. RIG=radiologically inserted gastrostomy. PIG=per-oral image-guided gastrostomy. NA=not applicable.
Table 4.
Postprocedural complications
| PEG | RIG | PIG | Total | p value | |
|---|---|---|---|---|---|
| Infection | 20/129 (16%) | 21/96 (22%) | 3/25 (12%) | 44/250 (18%) | 0·745 |
| Granulation tissue | 15/129 (12%) | 19/96 (20%) | 3/25 (12%) | 37/250 (15%) | 0·214 |
| Pain | 25/129 (19%) | 34/96 (35%) | 10/25 (40%) | 69/250 (28%) | 0·010 |
| Anxiety | 10/129 (8%) | 24/96 (25%) | 1/25 (4%) | 35/250 (14%) | 0·001 |
| Nausea | 12/129 (9%) | 10/96 (10%) | 2/25 (8%) | 24/250 (10%) | 0·923 |
| Diarrhoea | 6/129 (5%) | 10/96 (10%) | 3/25 (12%) | 19/250 (8%) | 0·185 |
| Pneumonia | 4/129 (3%) | 4/96 (4%) | 4/25 (16%) | 12/250 (5%) | 0·021 |
| Constipation | 16/129 (13%) | 22/96 (24%) | 9/25 (36%) | 47/250 (19%) | 0·010 |
| Fatigue | 15/129 (12%) | 23/96 (24%) | 4/25 (16%) | 42/250 (17%) | 0·050 |
Numbers are patients who experienced each event (n)/total patients in each group (N). The postprocedural period is the first 3 months after the completion of the gastrostomy procedure. PEG=percutaneous endoscopic gastrostomy. RIG=radiologically inserted gastrostomy. PIG=per-oral image-guided gastrostomy.
Valid weight measurements at 3 months after gastrostomy were collected for 170 (53%) of 323 patients, owing to attrition and difficulty in obtaining weight measurements from wheelchair-bound patients. After gastrostomy insertion, 43 (25%) of 170 patients gained more than 1 kg compared with weight at gastrostomy, 43 (25%) had loss or gain of 1 kg or less compared with weight at gastrostomy, and 84 (49%) lost more than 1 kg compared to weight at gastrostomy. The method of gastrostomy insertion did not influence the bodyweight post procedure (χ2, n=170; p=0·082). In the 43 patients who gained weight, these gains were small (median weight gain compared with weight at gastrostomy 3·1 kg, IQR 1·8–6·5). Appendix p 6 shows the nutritional outcome for patients in terms of weight at 3 months compared with weight at diagnosis. Continuing weight loss at 3 months after gastrostomy was associated with poor survival (appendix p 2).
The differences between patient quality of life at baseline and 3 months after gastrostomy were not statistically significant (mean [SD] total MQOL score 6·3 [1·6] at baseline vs 6·4 [1·6] at 3 months; p=0·749; appendix p 2). However, the strain of caregiving activities had increased significantly for carers at 3 months after gastrostomy (mean [SD] total MCSI score 9·9 [6·4] at baseline vs 11·8 [6·5] at 3 months; p=0·001; appendix p 2).
The results of post-hoc multiple imputation and propensity score analyses of the survival endpoints suggested that our findings for 30-day mortality and predictors of survival were robust to both missing data and gastrostomy method preferences in the 24 participating sites (appendix pp 2, 3).
Discussion
In our study, 30-day mortality was similar for percutaneous endoscopic gastrostomy, radiologically inserted gastrostomy, or per-oral image-guided gastrostomy, indicating that the three methods were as safe as each other in relation to procedure risk. The results suggested that weight loss at gastrostomy could affect procedure outcome, although caution with interpretation is necessary because 30-day mortality was low in this cohort. Our data indicate that overall mortality after gastrostomy insertion is independent of the gastrostomy method and is driven by the patient age at the onset of amyotrophic lateral sclerosis and the percentage of weight loss from diagnosis to the timepoint of gastrostomy.
In terms of periprocedural complications, the three different methods of gastrostomy were similar apart from the increased rate of distress, related to procedure tolerance, experienced by patients who underwent percutaneous endoscopic gastrostomy. This finding can be explained by the nature of the percutaneous endoscopic gastrostomy procedure, during which the patient's throat is intubated with an endoscope and the gastrostomy tube is pulled through the mouth towards its placement site.20 Patients who underwent radiologically inserted gastrostomy had a significantly increased rate of gastrostomy tube-related complications. Perhaps this is not surprising, because radiologically inserted gastrostomy tubes are usually relatively narrow in diameter (10–14 Fr), have a balloon-retention system (balloons could burst or deflate, causing gastrostomy tubes to migrate or fall out), and are not as securely fixed as those inserted by percutaneous endoscopic gastrostomy or per-oral image-guided gastrostomy.
In terms of the nutritional outcome for the patient, gastrostomy feeding prevented further weight loss in only about half of the patients. In the 43 (25%) patients who gained weight, these gains were small and of doubtful clinical benefit. Continuing weight loss at 3 months after gastrostomy was associated with poor survival. The nutritional data suggested that the greater the percentage of weight loss at the time of gastrostomy from diagnosis, the less likely it was for patients to recover this loss after gastrostomy. This finding was more evident for patients who at the time of gastrostomy had had more than 10% loss of their diagnosis weight; this subgroup of patients had also a significantly shorter survival compared with those who had lost up to 10% of their diagnosis weight. These results suggest that patients might benefit from early gastrostomy, before substantial weight loss that might not be reversible.
The reasons for the fairly poor nutritional outcome that we noted need further investigation. Perhaps weight loss due to continued denervation-induced skeletal muscle atrophy is masking nutritional benefits,21,22 which could be related to the change in metabolic state. Patients with amyotrophic lateral sclerosis can present hypermetabolism, and the caloric requirements of patients after gastrostomy might have been underestimated such that their energy intake was lower than energy expenditure.23 A small phase 2 study showed a potential benefit in terms of survival and nutritional gains for patients fed high calorific diets through a percutaneous endoscopic gastrostomy tube.24 Therefore, further study and subsequent evidence-based guidance on nutritional management post gastrostomy tube insertion is needed. A further potential metabolic explanation for our findings is related to the concept of refractory cachexia. The body of a patient with cachexia (defined as weight loss of more than 5%) is recognised to undergo irreparable metabolic changes, making artificial nutritional support ineffective.25 This idea is well recognised in oncology but has not been explored in patients with amyotrophic lateral sclerosis.
The effect of gastrostomy on the quality of life of patients in our study seemed to be neutral. Conversely, the strain of caregiving activities increased significantly after gastrostomy, although this was independent of insertion method. However, consequences of amyotrophic lateral sclerosis including increasing motor disability and dependency might contribute to caregiver strain. These results highlight the importance of provision of information and support from health-care professionals to carers, as well as to patients, before and after gastrostomy.
This study has limitations. This study was not a randomised controlled trial and the assignment of patients to a specific gastrostomy method was not done at random, but based on practical and clinical considerations. Therefore we can make associations but we are limited in the ability to draw conclusions with regard to the direct effects of gastrostomy on survival and nutritional outcome compared with not having had a gastrostomy. Another limitation is that, of 484 patients who had been referred for a gastrostomy in the 24 participating centres, we recruited 345 patients (participation rate 71%). Patient refusal and several logistical issues hindered full recruitment. Unfortunately, we could not obtain meaningful information for the potential participants who were not recruited to our study because we did not have the consent of these patients to do so, and we could not compare their characteristics with those of patients in this study. Practical difficulties in obtaining weight measurements at 3 months after gastrostomy introduced another limitation. The prospective element of this study allowed us to follow up a large number of patients after gastrostomy insertion and to consistently collect data related to the predetermined primary and secondary outcomes. A major strength of this study is that our sample is representative of the wider amyotrophic lateral sclerosis population: the baseline characteristics of the patients who took part are very similar to those of other reported cohorts of patients with amyotrophic lateral sclerosis.19,26
We noted significantly worse respiratory impairment in the per-oral image-guided gastrostomy group. Despite this, 30-day mortality was similar to the other groups. This observation would suggest that percutaneous endoscopic gastrostomy might be the optimum method of gastrostomy when respiratory function is largely unimpaired and per-oral image-guided gastrostomy when respiratory function is significantly compromised. Both percutaneous endoscopic gastrostomy and per-oral image-guided gastrostomy seemed to offer easier post-insertion tube management than radiologically inserted gastrostomy; ease of management is crucial, especially in very frail patients who undergo gastrostomy late, when they are more likely to feel the burden of other consequences of amyotrophic lateral sclerosis, such as respiratory problems and the loss of mobility and speech.
Our study showed that delay might lead to diminishing gains, especially for patients who at the time of gastrostomy have experienced excessive weight loss from their diagnosis weight. From a safety and efficacy perspective, the current guidelines of 10% weight loss might not be ideal and perhaps a better threshold would be to recommend gastrostomy insertion at a threshold similar to the one for cachexia—ie, at roughly 5% weight loss. Another recently suggested approach is to consider gastrostomy based on the ability of an individual to meet their total daily energy requirements.27 Delay of gastrostomy until after weight loss of more than 10% might convey minimal clinically meaningful benefit. However, some patients will not wish to undergo early gastrostomy. For such patients, gastrostomy will still have a role alleviating the difficulties caused by advanced dysphagia—eg, to allow administration of drugs and hydration—but in view of the possible diminishing nutritional benefits of delayed gastrostomy, other options of palliative support should also be considered.
Correspondence to: Dr Christopher J McDermott, Sheffield Institute for Translational Neuroscience, The University of Sheffield, 385A Glossop Road, Sheffield, S10 2HQ, UK c.j.mcdermott@sheffield.ac.uk
Acknowledgments
Acknowledgments
ProGas was funded by the Motor Neurone Disease Association of England, Wales, and Northern Ireland and the Sheffield Institute for Translational Neuroscience. We are very grateful to the patients and carers who participated in this study and to our funders for making this research possible.
Contributors
CJM was the chief investigator and study manager, helped develop the protocol and all study material, assessed patient eligibility, helped to recruit participants, participated in data collection at the principal site, (the Sheffield MND Care and Research Centre for Motor Neurone Disorders) advised on the conduct of the study, participated in data analysis, helped to interpret the results, and revised the manuscript. PJS was the principal investigator at the lead site (the Sheffield MND Care and Research Centre for Motor Neurone Disorders), helped to develop the protocol and all study material, assessed patient eligibility, helped to recruit participants, participated in data collection at the principal site, advised on the conduct of the study, participated in data analysis, helped to interpret the results, and revised the manuscript. TS was a coinvestigator and the study coordinator, helped develop the protocol and all study material, helped to recruit participants, participated in data collection at the principal site, facilitated recruitment and data collection from all other sites, created and updated the study database, conducted the data analysis, helped to interpret the results, and drafted and revised the manuscript. SJW was the statistician who advised on statistical matters, helped to conduct the data analysis, and revised the manuscript. AA-C, SC, FC, DD, CD, PE, MF, CG, GG, HH, COH, MJ, TM, AM, KM, RO, AP, AR, MR, KT, MRT, TW, CY were the principal investigators in the other ProGas sites, helped to develop the protocol, assess patient eligibility, recruit participants, collect data at their sites, advise on interpreting the results, and revise the manuscript.
ProGas Writing Committee
Christopher J McDermott, Pamela J Shaw, Theocharis Stavroulakis, Stephen J Walters, Ammar Al-Chalabi, Siddharthan Chandran, Francesca Crawley, David Dick, Colette Donaghy, Penelope Eames, Mark Fish, Carol Gent, George Gorrie, Hisham Hamdalla, C Oliver Hanemann, Michael Johnson, Tahir Majeed, Andrea Malaspina, Karen Morrison, Richard Orrell, Ashwin Pinto, Aleksandar Radunovic, Mark Roberts, Kevin Talbot, Martin R Turner, Timothy Williams, Carolyn Young.
Declaration of interests
CJM, PJS, AA-C, KT, and MRT are supported by the EU Joint Programme—Neurodegenerative Disease Research (JPND), UK Medical Research Council, and Economic and Social Research Council. PJS is supported as a National Institute for Health Research (NIHR) Senior Investigator. AA-C receives salary support from the NIHR Dementia Biomedical Research Unit at South London and Maudsley NHS Foundation Trust and King's College London and from the European Community's Health Seventh Framework Programme (FP7/2007–2013; grant agreement number 259867). The other authors declare no competing interests.
Supplementary Material
References
- 1.McDermott CJ, Shaw PJ. Diagnosis and management of motor neurone disease. BMJ. 2008;336:658–662. doi: 10.1136/bmj.39493.511759.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirker FJ, Oliver DJ. The development and implementation of a standardized policy for the management of dysphagia in motor neurone disease. Palliat Med. 2003;17:322–326. doi: 10.1191/0269216303pm748oa. [DOI] [PubMed] [Google Scholar]
- 3.Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17:139–146. doi: 10.1007/s00455-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 4.Hughes T. Neurology of swallowing and oral feeding disorders: assessment and management. J Neurol Neurosurg Psychiatry. 2003;74:48–52. doi: 10.1136/jnnp.74.suppl_3.iii48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skelton J. Nursing in the multidisciplinary management of motor neurone disease. Br J Nurs. 2005;14:20–24. doi: 10.12968/bjon.2005.14.1.17367. [DOI] [PubMed] [Google Scholar]
- 6.Squires N. Dysphagia management for progressive neurological conditions. Nurs Stand. 2006;20:53–57. doi: 10.7748/ns2006.03.20.29.53.c4108. [DOI] [PubMed] [Google Scholar]
- 7.Miller RG, Jackson CE, Kasarskis EJ. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stavroulakis T, Walsh T, Shaw PJ, McDermott CJ. Gastrostomy use in motor neurone disease (MND): a review, meta-analysis and survey of current practice. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:96–104. doi: 10.3109/17482968.2012.723722. [DOI] [PubMed] [Google Scholar]
- 9.Gordon PH, Mitsumoto H. Symptomatic therapy and palliative aspects of clinical care. In: Eisen AA, Shaw PJ, editors. Handbook of Clinical Neurology. Elsevier; Edinburgh: 2007. pp. 389–424. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld J, Ellis A. Nutrition and dietary supplements in motor neuron disease. Phys Med Rehabil Clin N Am. 2008;19:573–589. doi: 10.1016/j.pmr.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzberg HD, Benatar M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD004030.pub3. CD004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsumoto H, Davidson M, Moore D. Percutaneous endoscopic gastrostomy (PEG) in patients with ALS and bulbar dysfunction. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:177–185. doi: 10.1080/14660820310011728. [DOI] [PubMed] [Google Scholar]
- 13.Radunovic A, Mitsumoto H, Leigh PN. Clinical care of patients with amyotrophic lateral sclerosis. Lancet Neurol. 2007;6:913–925. doi: 10.1016/S1474-4422(07)70244-2. [DOI] [PubMed] [Google Scholar]
- 14.Andersen PM, Borasio GD, Dengler R. EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. Eur J Neurol. 2005;12:921–938. doi: 10.1111/j.1468-1331.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 15.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Disease El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 16.Cedarbaum JM, Stambler N, Malta E. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III) J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SR, Mount BM, Strobel MG, Bui F. The McGill quality of life questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability Palliat Med. 1995;9:207–219. doi: 10.1177/026921639500900306. [DOI] [PubMed] [Google Scholar]
- 18.Thornton M, Travis SS. Analysis of the reliability of the modified caregiver strain index. J Gerontol B Psychol Sci Soc Sci. 2003;58:S127–S132. doi: 10.1093/geronb/58.2.s127. [DOI] [PubMed] [Google Scholar]
- 19.Gordon PH, Cheng B, Salachas F. Progression in ALS is not linear but is curvilinear. J Neurol. 2010;257:1713–1717. doi: 10.1007/s00415-010-5609-1. [DOI] [PubMed] [Google Scholar]
- 20.Laasch HU, Wilbraham L, Bullen K. Gastrostomy insertion: comparing the options–PEG, RIG or PIG? Clin Radiol. 2003;58:398–405. doi: 10.1016/s0009-9260(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins TM, Burness C, Connolly DJ. A prospective pilot study measuring muscle volumetric change in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:414–423. doi: 10.3109/21678421.2013.795597. [DOI] [PubMed] [Google Scholar]
- 22.Bongers KS, Fox DK, Ebert SM. Skeletal muscle denervation causes skeletal muscle atrophy through a pathway that involves both Gadd45a and HDAC4. Am J Physiol Endocrinol Metabol. 2013;305:E907–E915. doi: 10.1152/ajpendo.00380.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genton L, Viatte V, Janssens JP, Heritier AC, Pichard C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clin Nutr. 2011;30:553–559. doi: 10.1016/j.clnu.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Wills AM, Hubbard J, Macklin EA. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014;383:2065–2072. doi: 10.1016/S0140-6736(14)60222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon K, Strasser F, Anker SD. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 26.Turner MR, Scaber J, Goodfellow JA, Lord ME, Marsden R, Talbot K. The diagnostic pathway and prognosis in bulbar-onset amyotrophic lateral sclerosis. J Neurol Sci. 2010;294:81–85. doi: 10.1016/j.jns.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Kasarskis EJ, Mendiondo MS, Matthews DE. Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. Am J Clin Nutr. 2014;99:792–803. doi: 10.3945/ajcn.113.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.