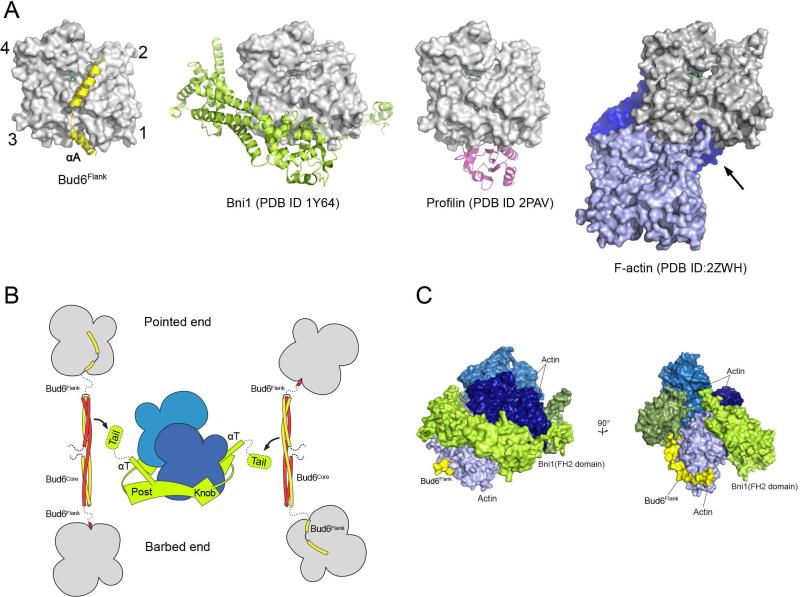
Figure 4. Mechanistic implications of the Bud6 structure and mode of actin binding.
A, The Bud6flank binding site on actin overlaps with that of other actin-assembly factors and is partially blocked in F-actin. Crystal structures of Bud6flank, profilin, and the Bni1 FH2 domain in complex with actin are shown in the same orientation. The barbed-end groove occupied by helix αA in Bud6 is also part of the binding surface for profilin and the FH2 domain. The groove is also blocked in F-actin by the DNAse I binding loop (arrow) of a longitudinally apposed subunit (medium blue) in the helical filament. Three actin subunits of a filament are drawn based on the X-ray fiber diffraction structure of F-actin (PDB ID 2ZWH); examination of a cryo-electron microscopy reconstruction leads to the same conclusion (PDB ID 3MFP, not shown). B, Schematic summary of available structural information for the Bni1 FH2 domain and C-Bud6 and their interactions with actin. Components are drawn approximately to scale, and the illustration is based on structures of the Bni1 FH2 domain (green) bound to actin (blue), the Bud6core domain (yellow and red), and Bud6flank in complex with actin (yellow or red, with actin in gray). No structure is available for Bud6core in complex with Bni1, but biochemical studies map the binding interaction to the “tail” of Bni1, which lies just C-terminal to the long “αT” helix of the FH2 domain. The Bni1 tail and the ~20 residue linker that connects Bud6 flank and core domains are shown as dotted lines, because they are not present in available crystal structures. The Bni1 FH2 dimer is thought to promote nucleation by bridging between two or more actin subunits in a filament-like orientation, via contacts of its “knob” and “post” elements. C, Superposition of Bud6flank on a Bni1/actin complex. Three actin subunits and a Bni1 FH2 domain dimer from the crystal structure of the complex (PDB ID 1Y64) are shown in shades of blue and green, respectively. The interaction with the formin arranges the actin subunits in a filament-like orientation that is proposed to lead to formation of a nascent filament (Otomo et al., 2005b). Bud6flank (yellow) is docked based on superposition of the present structure with the light-blue actin subunit. The Bud6 binding site on the medium and dark blue actin subunits is blocked by contact with the FH2 domain, but it is accessible on the light blue subunit, which is in contact with only the post-site of the FH2 domain. We speculate that this mode of interaction could allow Bud6 to contribute to filament nucleation by the FH2 domain (see text). There is a modest steric clash between the end of Helix αB in Bud6 and the opposite subunit in the FH2 dimer (rotated view), but the precise orientation of the two FH2 subunits that leads to this clash arises from crystallographic symmetry and is not thought to be directly relevant to Bni1-mediated nucleation. Note that one of the flexible linkers connecting the two halves of the FH2 dimer is not illustrated; due to an artifact in the crystal structure, it connects to an adjacent FH2 subunit in the lattice rather than closing the FH2 dimer.