Table 1.
Relative energies with respect to free CO2 and free cobalt(III)–salen complex of the precursor, transition and product state of the CO2 insertion reaction as shown in Figure 4.
| Salen ligand | trans-Ligand | Precursor statea (preS) |
Transition statea (TS) |
Product statea (PS) |
Activation barrier | Reaction energy |
| [kJ·mol−1] | [kJ·mol−1] | [kJ·mol−1] | [kJ·mol−1] | [kJ·mol−1] | ||
| 1 | chloride | −14 | 31 | −47 | 45 | −33 |
| 1 | CH3C(O)O− | −14 | 45 | −30 | 59 | −16 |
| 1ab | CH3C(O)O− | −12 | 41 | −31 | 53 | −19 |
| 1bc | CH3C(O)O− | −11 | 39 | −37 | 50 | −26 |
| 1 | p-methoxyphenolate | −14 | 41 | −33 | 55 | −19 |
| 1 | CCl3C(O)O− | −21 | 34 | −30 | 55 | −9 |
| 1 | 2,4-dinitrophenolate | −22 | 68 | −29 | 90 | −7 |
| 1 | 2,4,6-trinitrophenolate | −11 | 79 | −16 | 90 | −5 |
| 1 | TBDd | −14 | 65 | −29 | 79 | −15 |
| 1 | none | −13 | 58 | −6 | 71 | 7 |
| 1 | ethylene oxide | −17 | 100 | −8 | 117 | 9 |
aPotential energies relative to free CO2 and the free cobalt(III)–salen complex, i.e., the reactant state was set to zero for each trans-ligand L.
bSalen with cyclohexyl backbone, 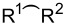 = -C4H8-, R3–6 = H
= -C4H8-, R3–6 = H
cSalen with cyclohexyl backbone, 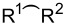 = -C4H8-, R3–6 = t-Bu
= -C4H8-, R3–6 = t-Bu
dTBD = 1,5,7-triazabicyclo[4.4.0]dec-5-ene.
