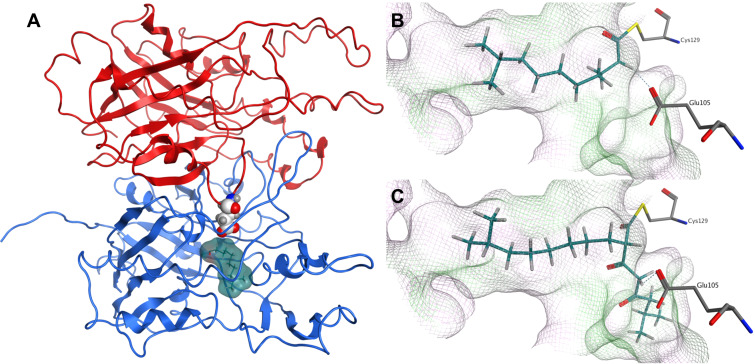
Figure 2.
Dimeric structure of modeled PpyS (A). Chain A (blue), chain B (red). 14 (surface, cyan) is covalently docked to the active site at Cys129α and the atoms of Glu105β are represented as white spheres. Glu105 is proposed to act as a catalytic base. A detailed view of the proposed PpyS-binding pocket with covalently docked 14 (B) and 16 (C), respectively. The cavity of the binding pocket is shown in a line representation, where green represents a lipophilic, magenta a hydrophilic and white a neutral surface area. Possible hydrogen bonds are shown as dashed blue lines.