Figure 2.
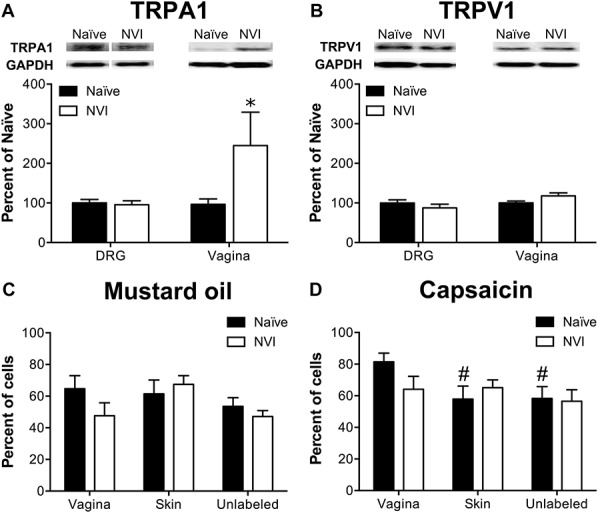
NVI increased TRPA1 protein expression in the vagina but not in primary sensory neurons innervating the vagina. (A) Representative Western blots are shown for TRPA1 and corresponding GAPDH protein expression with bands at 127 and 35 kD, respectively, in both DRG and vagina from naive and NVI mice. TRPA1 protein expression was significantly increased in vagina, but not DRG, from NVI mice compared with naive mice. (B) Representative Western blots are shown for TRPV1 and corresponding GAPDH protein expression with bands at 85 and 35 kD, respectively, in both DRG and vagina from naive and NVI mice. TRPV1 protein expression was not significantly different in NVI DRG or vagina compared with naive counterparts. Calcium imaging was performed on lumbosacral (L5-S1) DRG neurons retrogradely labeled by injection of Alexa Fluor–conjugated cholera toxin-β (CTB) into the distal vagina, 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) into the perivaginal skin, and adjacent unlabeled DRG to measure responses to 100 μM mustard oil (MO; C) and 1 μM capsaicin (D). (C) No significant difference in functional TRPA1 expression, measured as the percentage of MO-responsive DRG neurons, was observed between naive and NVI mice for any population of DRG neurons tested. (D) Only in naive mice, DRG neurons back-labeled from the vagina were significantly more likely to respond to 1 μM capsaicin, suggesting greater functional TRPV1 expression, compared with those back-labeled from the perivaginal skin or unlabeled DRG. When compared across both agonists, vagina-specific DRG neurons from NVI mice had a significantly reduced percentage of responsive neurons compared with naive (P < 0.05; 2-way ANOVA). (A and B): naive, DRG: n = 10, vagina: n = 5; NVI, DRG: n = 8, vagina: n = 4; (C): naive, n = 8, NVI, n = 10; (D): naive, n = 8; NVI, n = 6. *P < 0.05 vs naive, #P < 0.05 vs vagina; 2-way ANOVA and Bonferroni's posttest.
