Abstract
BACKGROUND
A recent clinical trial suggests that printed (PDS) and computer decision support (CDS) interventions are safe and effective in reducing antibiotic use in acute bronchitis relative to usual care (UC).
OBJECTIVE
Our aim was to evaluate the cost-effectiveness of decision support interventions in reducing antibiotic use in acute bronchitis.
DESIGN
We conducted a clinical trial-based cost-effectiveness analysis comparing UC, PDS and CDS for management of acute bronchitis. We assumed a societal perspective, 5-year program duration and 30-day time horizon.
PATIENTS
The U.S. population aged 13–64 years presenting with acute bronchitis in the ambulatory setting.
INTERVENTIONS
Printed and computer decision support interventions relative to usual care.
MAIN MEASURES
Cost per antibiotic prescription safely avoided.
KEY RESULTS
In the base case, PDS dominated UC and CDS, with lesser total costs (PDS: $2,574, UC: $2,768, CDS: $2,805) and fewer antibiotic prescriptions (PDS: 3.79, UC: 4.60, CDS: 3.95) per patient over 5 years. In one-way sensitivity analyses, PDS dominated UC across all parameter values, except when antibiotics reduced work loss by ≥ 1.9 days or the probability of hospitalization within 30 days was ≥ 0.9 % in PDS (base case: 0.2 %) or ≤ 0.4 % in UC (base case: 1.0 %). The dominance of PDS over CDS was sensitive both to probability of hospitalization and plausible variation in the adjusted odds of antibiotic use in both strategies.
CONCLUSIONS
A PDS strategy to reduce antibiotic use in acute bronchitis is less costly and more effective than both UC and CDS strategies, although results were sensitive to variation in probability of hospitalization and the adjusted odds of antibiotic use. This simple, low-cost, safe, and effective intervention would be an economically reasonable component of a multi-component approach to address antibiotic overuse in acute bronchitis.
KEY WORDS: decision support, antibiotics, acute bronchitis, cost effectiveness
INTRODUCTION
Antibiotics are prescribed in > 50 % of ambulatory visits for colds, upper respiratory tract infections and acute bronchitis, despite the predominantly viral etiology of these conditions.1,2 This antibiotic overuse is concerning because there is little evidence that it provides any patient benefit and there is strong evidence that it contributes to the growth of antibiotic resistance among common bacterial pathogens.3–6
Addressing ambulatory antibiotic overuse for acute respiratory tract infections remains a major challenge. Researchers have deployed an array of interventions to improve antibiotic targeting and reduce antibiotic overuse; these have only modestly impacted antibiotic prescribing behavior.7 In general, these interventions—which include physician and patient education, physician reminders, physician audit and feedback, delayed antibiotic prescriptions, financial incentives and various combinations of the above—appear to reduce antibiotic overuse by approximately 10 %.8 Yet, even modestly effective interventions may be economically reasonable for use if implementation costs are low.9 An intervention that is safe, at least modestly effective, low cost and feasible for wide deployment has the potential to be a cost-effective component of a multi-component quality improvement approach to reduce antibiotic overuse in the ambulatory setting.
A recent randomized controlled clinical trial suggests that printed decision support (PDS) and computer decision support (CDS) strategies deployed in ambulatory settings are safe and effective in reducing antibiotic use in ambulatory management of acute bronchitis in adolescents and adults, relative to usual care (UC).10 Yet, little is known about the cost-effectiveness of these strategies. Here, we evaluate the cost-effectiveness of PDS and CDS strategies for safely reducing antibiotic use in ambulatory management of acute bronchitis.
METHODS
Using TreeAge Pro 2015 software (TreeAge Software Inc., Williamstown, MA), we developed a clinical-trial-based cost-effectiveness analysis in which we evaluated two strategies for safely reducing antibiotic use in ambulatory management of acute bronchitis relative to UC: PDS and CDS.10 We assumed a societal perspective, 5-year program duration and 30-day time horizon from the time of each office visit. Our cohort was the U.S. population aged 13–64 years presenting in the ambulatory setting with acute cough illness and diagnosed with acute uncomplicated bronchitis.10 We compared strategies in terms of cost per antibiotic prescription safely avoided, a previously described outcome measure that is particularly useful in evaluating the cost-effectiveness of interventions to improve the appropriateness of antibiotic prescribing where the primary goal is to reduce antibiotic use without negatively impacting patients’ quality or quantity of life.9
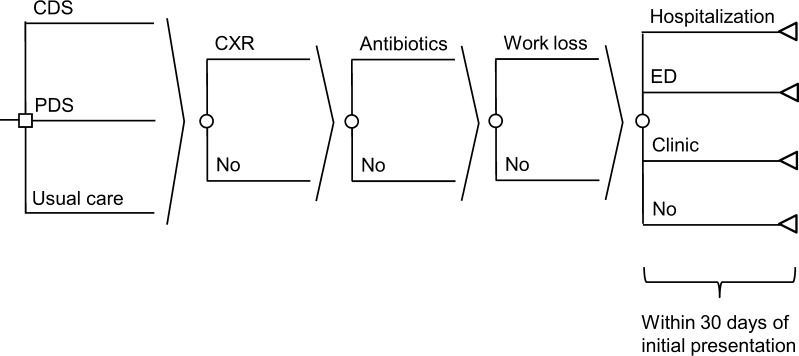
The model structure was identical across all three strategies (Fig. 1). We assumed that each patient presenting to the office was first evaluated clinically prior to receiving a chest x-ray (or not), receiving an antibiotic prescription (or not) and benefitting from a reduction in days of work loss (or not) if prescribed antibiotics. We assumed that patients could return to clinic, return to the emergency department, be hospitalized, or seek no further care within 30 days of the initial presentation. The primary difference between the three strategies was the added cost of the interventions and reduced likelihood of an antibiotic prescription in the PDS and CDS strategies relative to UC.
Figure 1.
Cost-effectiveness model comparing usual care, printed decision support, and computer decision support strategies for safely reducing antibiotic use in acute bronchitis. CDS: computer decision support, PDS: printed decision support, CXR: chest x-ray, ED: emergency department. For sake of brevity, each probability node (depicted as a circle) is assumed to be attached in duplicate to each branch to the left of that probability node.
In this randomized control-trial-based cost-effectiveness analysis, we drew parameter values from a recent trial conducted in a fully integrated healthcare delivery system located in rural Pennsylvania, in an effort to replicate the cost-effectiveness of UC, PDS and CDS strategies as deployed in a real world setting (Table 1).10 In the baseline year prior to intervention deployment, we assumed no difference in the probability of an empiric antibiotic prescription (75 %) across the three strategies, despite differences in the baseline probability of an empiric antibiotic prescription in the trial. We made this modeling choice in an effort to eliminate the impact of baseline differences in practices’ antibiotic prescribing behavior on the cost-effectiveness of the three strategies, doing so based on the use of trial-based odds ratios that accounted for baseline antibiotic prescribing differences.10 We assumed that the adjusted odds of antibiotic use during each of the 5 years of the program relative to the baseline year equaled 1.10 (range: 0.85–1.43) in the UC strategy, 0.57 (range: 0.40–0.82) in the PDS strategy and 0.64 (range: 0.45–0.91) in the CDS strategy, based on the adjusted trial-based odds of antibiotic use at the end of the intervention’s first year relative to the baseline year.10 With a 75 % base case probability of antibiotic prescription, those odds ratios represent the following probabilities of antibiotic prescription in the model: UC 76.7 %, PDS 63.1 %, CDS 65.7 %. We assumed no incremental reductions in the adjusted odds of antibiotic use in the 5 years of the program beyond those reported after the first year, an assumption that may bias the analysis against the PDS and CDS strategies if there are further reductions in antibiotic overuse in later years, or bias the analysis in favor of these strategies if there is regression to baseline antibiotic overuse in later years. We modeled differences in the probabilities of chest radiography, return to clinic within 30 days, emergency department visit within 30 days, and hospitalization within 30 days across the three strategies based on clinical trial data (Table 1).
Table 1.
Parameter Values for Base Case, One-Way and Probabilistic Sensitivity Analyses
| Description | Base | Range | Distribution | Source |
|---|---|---|---|---|
| Probabilities | ||||
| Empiric antibiotics in yr 0 prior to intervention | 75 % | Not varied | Not varied | 10 |
| Adjusted odds of antibiotics during intervention | ||||
| Relative to baseline, yrs 1–5 | ||||
| Usual care (UC) | 1.10 | 0.85–1.43 | Uniform | 10 |
| Printed decision support (PDS) | 0.57 | 0.40–0.82 | Uniform | 10 |
| Computerized decision support (CDS) | 0.64 | 0.45–0.91 | Uniform | 10 |
| Chest x-ray | ||||
| UC | 4.2 % | 0 %–10 % | Beta | 10 |
| PDS | 3.2 % | 0 %–10 % | Beta | 10 |
| CDS | 4.9 % | 0 %–10 % | Beta | 10 |
| Return to clinic within 30-days | ||||
| UC | 7.6 % | 0 %–15 % | Beta | 10 |
| PDS | 7.1 % | 0 %–15 % | Beta | 10 |
| CDS | 8.3 % | 0 %–15 % | Beta | 10 |
| Emergency department within 30 days | ||||
| UC | 0.2 % | 0–3 % | Beta | 10 |
| PDS | 1.2 % | 0–3 % | Beta | 10 |
| CDS | 0.0 % | 0–3 % | Triangular | 10 |
| Hospitalization, within 30 days | ||||
| UC | 1.0 % | 0–3 % | Beta | 10 |
| PDS | 0.3 % | 0–3 % | Beta | 10 |
| CDS | 1.1 % | 0–3 % | Beta | 10 |
| Durations | ||||
| Hours of education per physician, PDS and CDS, yr 1 | 1.0 | 0.5–1.5 | Triangular | 10 |
| Days of work loss due to illness and seeking care, no antibiotics | 3.0 | Not varied | Not varied | 4, Estimate |
| Decrease in days of work loss with antibiotics | 0.0 | −1.0–1.0 | Gamma | 4, Estimate |
| Costs | ||||
| Physician education per hour, PDS and CDS, yr 1 | $120 | $80–$160 | Gamma | 12 |
| Educational brochure per patient, PDS and CDS, yrs 1–5 | $0.05 | $0.03–$0.30 | Gamma | 13 |
| Exam room poster per patient, PDS, yr 1 | $0.96 | $0.48–$5.76 | Gamma | 13 |
| Medical record programming per patient, CDS, yr 1 | $18 | $9–$27 | Gamma | 13 |
| Chest x-ray | $31 | $10–$100 | Gamma | 14 |
| Antibiotics | $30 | $5–$100 | Gamma | 15, Estimate |
| Work loss per day due to illness and seeking care | $136 | $96–$176 | Gamma | 16 |
| Clinic visit | $107 | $57–$157 | Gamma | 17 |
| Emergency department visit | $680 | $280–$1,080 | Gamma | 18 |
| Hospitalization | $5,424 | $4,424–$6,424 | Gamma | 19 |
We modeled direct medical and non-medical costs per established guidelines,11 and included the following costs: the PDS and CDS interventions, chest radiography, antibiotics, work loss due to illness, and clinic visits, emergency department visits, and hospitalizations within 30 days of presentation (Table 1). In the PDS intervention, we modeled the costs of physician education in year 1, PDS posters in year 1 and educational brochures for each patient across all years.10 We estimated base case exam room poster cost per patient by dividing the total cost of purchasing posters ($5 per poster × 177 posters), inflated to 2013 US dollars by the number of enrolled patients in the printed decision support during the intervention period (1001). In the CDS intervention, we modeled the costs of physician education in year 1, electronic medical record programming in year 1 and educational brochures for each patient across all years.10 Base case parameters values for PDS and CDS intervention costs were based on clinical trial expenditures (Table 1). We modeled the costs of physician education in the PDS and CDS strategies by assuming that each physician spent 1 h on educational activities in the first year of the intervention at a salary of $120 per hour.10,12 In the base case, we assumed no difference in duration of work loss due to illness between patients receiving and not receiving antibiotics based on evidence suggesting minimal impact of antibiotics on return to work, and relaxed this assumption in sensitivity analyses.4 All costs are listed in 2013 U.S. dollars with prior costs inflated using the U.S. Consumer Price Index.
We performed a base case cost-effectiveness analysis, one-way and probabilistic sensitivity analyses to examine the robustness of model results to parameter variation. In the probabilistic sensitivity analysis, we simultaneously varied all parameters over their maximum ranges, generally varying probabilities over beta distributions and durations, and costs over gamma distributions (Table 1).10,12–19 In traditional cost-effectiveness analyses, interventions are often compared in terms of cost per quality-adjusted life year (QALY) saved, and the societal willingness-to-pay (WTP) threshold is often assumed to be $50,000–$100,000 per QALY saved.20 In our analysis, which compared strategies in terms of cost per antibiotic safely avoided, we assumed that the societal WTP threshold equaled $43 per antibiotic safely avoided, based on prior estimates of the downstream societal costs of antibiotic resistance attributable to an antibiotic prescription.9 The underlying concept is that society should be willing to pay $43 to safely avoid an antibiotic prescription, because the total downstream societal costs of antibiotic resistance attributable to an antibiotic prescription equal $43.
RESULTS
In the base case analysis, the PDS strategy dominated both the UC and CDS strategies, with lesser total costs (PDS: $2,574, UC: $2,768, CDS: $2,802) and fewer antibiotic prescriptions (PDS: 3.79, UC: 4.60, CDS: 3.95; Table 2) per patient over 5 years.
Table 2.
Base Case Cost-Effectiveness Analysis of Three Strategies For Safely Reducing Antibiotic Use in the Ambulatory Management of Patients Aged 13–64 Years Presenting with Acute Bronchitis
| Strategy | Costa | Incr. Cost | Effectivenessb | Incr. Effectivenessc | ICERd |
|---|---|---|---|---|---|
| Printed Decision Support | $2,574 | 3.78 | |||
| Usual Care | $2,768 | $194 | 4.60 | −0.82 | Dominatede |
| Computer Decision Support | $2,802 | $34 | 3.94 | −0.16 | Dominatede |
aCumulative 5-year societal cost per five cases of acute bronchitis
bCumulative 5-year number of antibiotic prescriptions per five cases of acute bronchitis
cIncremental effectiveness; represents the incremental number of antibiotic prescriptions safely avoided
dIncremental cost-effectiveness ratio; represents the incremental societal cost per antibiotic prescription safely avoided
eDominated: other strategies are more effective and less costly (i.e., Printed Decision Support)
In one-way sensitivity analyses, the PDS strategy dominated the UC strategy across all tested parameter values, except when antibiotics reduced the duration of work loss by ≥ 1.9 days (base case: 0.0 days), or the probability of hospitalization within 30 days was ≥ 0.9 % in the PDS strategy (base case: 0.2 %) or ≤ 0.4 % in the UC strategy (base case: 1.0 %; Table 3). The PDS strategy dominated the CDS strategy across all tested parameter values, except when the adjusted odds of antibiotic use during the intervention period relative to baseline was ≥ 0.64 in the PDS strategy (base case: 0.57) or ≤ 0.57 in the CDS strategy (base case: 0.64), or the probability of hospitalization within 30 days was ≥ 0.9 % in the PDS strategy (base case: 0.2 %) or ≤ 0.4 % in the CDS strategy (base case: 1.1 %). The threshold parameter values for the adjusted odds of antibiotic use in the PDS and CDS strategies were considered plausible in the setting of clinical trial data, suggesting no significant difference in the adjusted odds of antibiotic use between PDS and CDS strategies. The PDS strategy cost less than the societal WTP threshold of $43 per antibiotic safely avoided across all tested parameter values, except when the probability of hospitalization within 30 days was ≤ 0.2 % in the UC strategy (base case: 1.0 %), ≥ 1.0 % in the PDS strategy (base case: 0.3 %) or ≤ 0.3 % in the CDS strategy (base case: 1.1 %). The analysis was not sensitive to variation in the costs of physician education or patient educational brochures in the PDS and CDS strategies, posters in the PDS strategy, or electronic medical programming in the CDS strategy. Nor was the analysis sensitive to the probabilities or costs of chest x-ray, return office visit, or emergency department visit.
Table 3.
One-way Sensitivity Analyses Showing Only those Parameters that, when Varied Across their Maximum Range, Caused the Printed Decision Support (PDS) Strategy to No Longer Dominate the Usual Care (UC) or Computer Decision Support (CDS) Strategies, or the Incremental Cost-Effectiveness Ratio of the PDS Strategy to be Greater than the Societal Willingness-to-Pay Threshold of $43 per Antibiotic Safely Avoided
| Parameter | Base case | PDS dominates | PDS ICER < $43 per antibiotic avoided | |
|---|---|---|---|---|
| UC | CDS | |||
| Adjusted odds of antibiotics use during | ||||
| Intervention relative to baseline period, yr 1–5 | ||||
| †PDS | 0.57 | Always | <0.64 | Always |
| ‡CDS | 0.64 | Always | >0.57 | Always |
| Decrease in days of work loss with antibiotics | 0.0 | <1.9 | Always | Always |
| Hospitalization, within 30 days | ||||
| *UC | 1.0 % | >0.4 % | Always | >0.2 % |
| †PDS | 0.3 % | <0.9 % | <0.9 % | <1.0 % |
| ‡CDS | 1.1 % | Always | >0.4 % | >0.3 % |
*UC usual care
†PDS printed decision support
‡CDS computer decision support
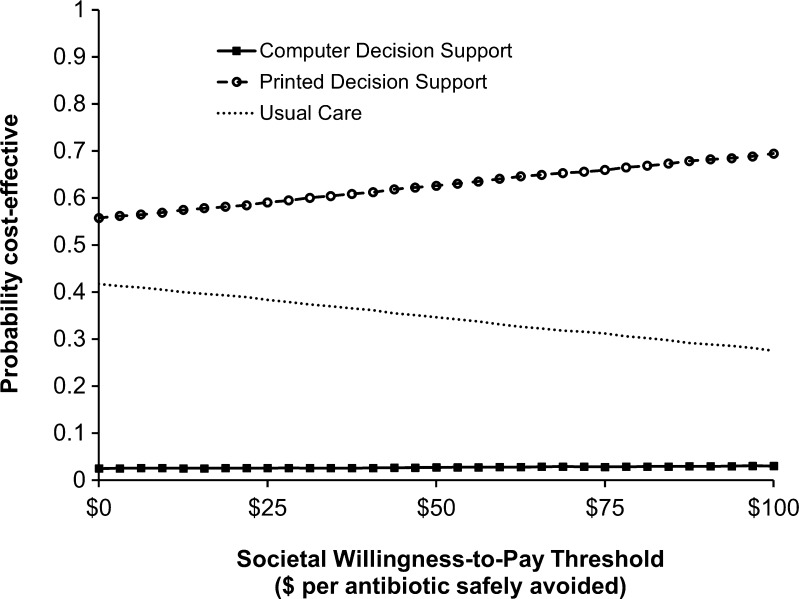
In a probabilistic sensitivity analysis, the probability of the PDS strategy being preferred at a societal WTP threshold of $43 per antibiotic safely avoided was 61.7 % (Fig. 2). The PDS strategy was preferred across all societal WTP thresholds from $0 to $100 per antibiotic safely avoided.
Figure 2.
Probabilistic sensitivity analysis comparing usual care, printed decision support, and computer decision support strategies for safely reducing antibiotic use in acute bronchitis. Results are presented in the form of an acceptability curve in which the net monetary benefits of all three strategies are compared simultaneously across a range of societal willingness-to-pay thresholds for each iterative trial. The x-axis depicts willingness-to-pay thresholds, representing the estimated downstream societal cost of antibiotic resistance per antibiotic prescription. The y-axis depicts the likelihood that strategies would be considered cost-effective for each willingness-to-pay threshold.
In the clinical trial upon which this analysis was based, no statistically significant differences were observed in antibiotic prescriptions, clinical resource utilization, or patient outcomes between the PDS and CDS strategies. In a secondary sensitivity analysis, we assumed no differences in the adjusted odds of antibiotic use in the PDS and CDS strategies (0.60 in both). Here, we found that PDS and CDS strategies were equally effective, with PDS costing $220 less than CDS per patient over 5 years ($2,580 vs. $2,800).
DISCUSSION
A PDS strategy to safely reduce antibiotic use in ambulatory management of acute bronchitis is less costly and more effective than UC and CDS strategies, although results were sensitive to plausible variation in probability of hospitalization and the adjusted odds of antibiotic use across strategies. This study adds to our understanding of the economic value of interventions to safely reduce antibiotic overuse in management of acute respiratory tract infections, such as acute bronchitis, where antibiotic overuse persists despite minimal evidence of patient benefit.4,8 Our results suggest that a simple, low cost, safe, and modestly effective intervention to reduce antibiotic overuse in acute bronchitis is likely to be cost saving. This finding is in concordance with prior work suggesting that ‘antibiotic-restrictive’ strategies for management of acute bronchitis21 and acute respiratory tract infections,9 in general, are likely to be cost-effective, provided that there is no impact of antibiotic restriction on clinical outcomes. Both the PDS and CDS interventions evaluated here compare favorably to education, audit and feedback and delayed antibiotic prescription interventions with regard to effectiveness, cost and ease of deployment.8 Given that the PDS strategy only reduced antibiotic overuse by 11.7 % and that antibiotics continue to be prescribed for > 70 % of patients presenting with acute bronchitis despite evidence of minimal clinical benefit, a PDS intervention is unlikely to be the definitive solution for the intractable problem of antibiotic overuse for acute bronchitis.4,8,10 Yet, in the setting of evidence suggesting that multi-component strategies are more effective than single-component strategies in reducing antibiotic overuse, our findings suggest that a PDS intervention would represent one economically reasonable component of a multi-component approach to address antibiotic overuse in the ambulatory setting.7
This analysis has several strengths. First, we found that the PDS strategy was preferred over UC, despite incorporating conservative assumptions that tended to bias the analysis in favor of UC. For instance, we chose not to model adverse events associated with antibiotic use, a modeling decision that would have biased the analysis in favor of strategies that reduce antibiotic use (i.e., the PDS and CDS strategies). In addition, we assumed no further incremental reductions in antibiotic overuse in later years of the intervention beyond those reported in the first year of the intervention in the trial.10 Second, our finding that the PDS strategy was preferred over UC was robust to parameter variation in sensitivity analyses. Finally, PDS was still preferred over CDS, based on lower cost, when no differences in effectiveness or outcomes were assumed between these two strategies.
This analysis also has limitations. First, because this cost-effectiveness analysis was based on results from a recent randomized controlled trial evaluating UC, PDS and CDS strategies in a fully integrated healthcare delivery system located in rural Pennsylvania with an existing electronic medical record system, our results may not be generalizable to other U.S. contexts where there are, for instance, different baseline levels of antibiotic prescribing or different legacy interventions already in place in an effort to reduce antibiotic overuse in management of acute respiratory tract infections such as acute bronchitis.10 Second, the cost-effectiveness of the PDS strategy was sensitive to the reduction in days of work loss associated with antibiotic use. The results of our one way sensitivity analysis suggest that the PDS strategy no longer dominated UC when antibiotics reduced the duration of work loss by ≥ 1.9 days. However, results from a recent meta-analysis evaluating the impact of antibiotic use on clinical outcomes in patients with acute bronchitis suggest that this antibiotic-associated reduction in the duration of work loss is relatively unlikely.4
In summary, this analysis suggests that a simple, low-cost, safe, and modestly effective PDS strategy to reduce antibiotic overuse in acute bronchitis is less costly and more effective in safely reducing antibiotic overuse in the management of acute bronchitis than UC and CDS strategies, although this finding was sensitive to plausible variation in probability of hospitalization and the adjusted odds of antibiotic use across strategies. Given the persistent problem of antibiotic overuse in the management of many self-limited, predominantly viral respiratory tract infections, and the greater effectiveness of multi-component interventions to reduce this antibiotic use, this analysis suggests that PDS should be considered an economically reasonable component of a multi-component approach to safely limit antibiotic overuse in ambulatory settings.
Acknowledgements
CIM’s work was supported by the National Institute of Health (T32 AG21885), the Doris Duke Charitable Foundation, and the University of Pittsburgh School of Medicine Clinical Scientist Training Program. KJS’s work was supported by the National Institute of Allergy and Infectious Diseases (R01AI076256). This research was presented at the annual meeting of Society of General Internal Medicine held in San Diego, 23–26 April 2014.
Conflicts of Interest
All authors report no conflicts of interest.
REFERENCES
- 1.Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA. 1997;278(11):901–904. doi: 10.1001/jama.1997.03550110039033. [DOI] [PubMed] [Google Scholar]
- 2.Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302(7):758–766. doi: 10.1001/jama.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis (review). Cochrane Database Syst Rev. 2010;CD000247. [DOI] [PubMed]
- 4.Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis (review). Cochrane Database Syst Rev. 2012;CD000245. [DOI] [PubMed]
- 5.Goosens H, Ferech M, Stichele RB, Elseviers S. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. doi: 10.1016/S0140-6736(05)70799-6. [DOI] [PubMed] [Google Scholar]
- 6.van de Sande-Bruinsma N, Grundman J, Verloo D, et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11):1722–1730. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2009;CD003539. [DOI] [PMC free article] [PubMed]
- 8.Linder JA. Antibiotic prescribing for acute respiratory tract infections—success that’s way off the mark. JAMA Intern Med. 2013;173(4):273–275. doi: 10.1001/jamainternmed.2013.1984. [DOI] [PubMed] [Google Scholar]
- 9.Michaelidis CI, Zimmerman RK, Nowalk MP, Fine MJ, Smith KJ. Cost-effectiveness of procalcitonin-guided therapy for outpatient management of acute respiratory tract infections in adults. J Gen Intern Med. 2014;29(4):579–586. doi: 10.1007/s11606-013-2679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173(4):267–273. doi: 10.1001/jamainternmed.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 12.Annual Salary, Physician – Internal Medicine, Salary.com. Available at http://swz.salary.com/SalaryWizard/Internist-Salary-Details.aspx. Accessed February 22, 2015.
- 13.Internal Cost Data, Institute for Advanced Application, Geisinger Health System, Danville, PA.
- 14.Centers for Medicare and Medicaid Services. Physician Fee Schedule, CPT 71020 (chest x-ray, two-view). Available at http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed February 22, 2015.
- 15.Mainous AG, Hueston WJ. The cost of antibiotics in treating upper respiratory tract infections in a Medicaid population. Arch Fam Med. 1998;7(Jan/Feb):45–49. doi: 10.1001/archfami.7.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Bureau of Labor Statistics. Median Hourly Wage, 2012. Available at http://www.bls.gov/oes/current/oes_nat.htm. Accessed February 22, 2015.
- 17.Centers for Medicare and Medicaid Services. Physician Fee Schedule, CPT 99214 (outpatient visit, estimated). Available at http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. February 22, 2015.
- 18.Mehrotra A, Liu H, Adams JL, et al. Comparing costs and quality of care at retails clinics with that of other medical settings for three common illnesses. Ann Intern Med. 2009;151(5):321–328. doi: 10.7326/0003-4819-151-5-200909010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HCUPnet. Mean U.S. Hospitalization Cost for Acute Bronchitis (ICD-9 466.0), Ages 18–84, 2011. Availableat http://hcupnet.ahrq.gov. Accessed February 22, 2015.
- 20.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 21.Hueston WJ. Antibiotics: neither cost effective nor ‘cough’ effective. J Fam Pract. 1997;44(3):261–265. [PubMed] [Google Scholar]
- 22.Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the US, 1996–2010. Poster, 2013 IDWeek Conference. [DOI] [PMC free article] [PubMed]