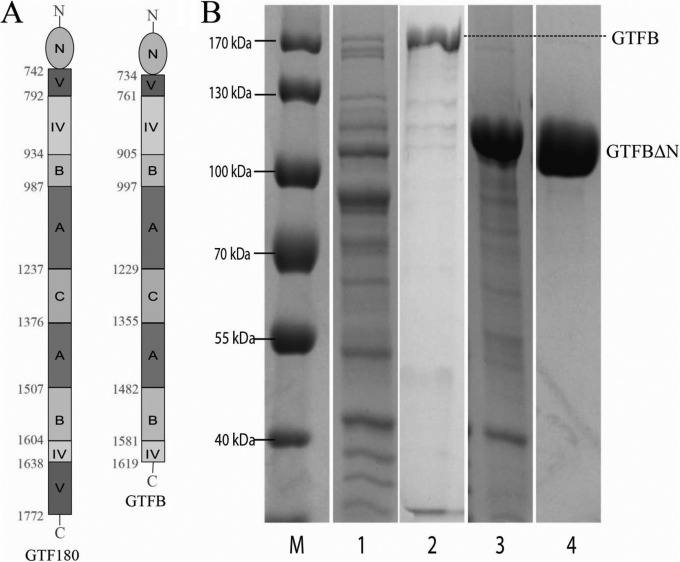
FIG 1.
(A) Linear schematic representation of the domain organization of full-length GTF180 of L. reuteri 180 and GTFB of L. reuteri 121 (with N-terminal variable region). Structural analysis of the L. reuteri 180 GTF180-ΔN protein has shown that its peptide chain follows a “U” path and that four of its five domains are built up from discontinuous N- and C-terminal parts of the peptide chain. Only the C domain is formed from one continuous stretch of amino acids (27, 40–42). The GTFB protein has a similar organization, except that the C-terminal part of domain V is absent. The amino acid residue numbers indicate the start and end of each domain. (B) SDS-PAGE (8%, Coomassie blue stained) analysis of GTFB and GTFB-ΔN expression. Lane 1, whole-cell extract of E. coli with pET15b-gtfB plasmid; lane 2, purified GTFB (predicted molecular mass, 179 kDa); lane 3, whole-cell extract of E. coli with pET15b-gtfB-ΔN plasmid; lane 4, purified GTFB-ΔN (predicted molecular mass, 99 kDa); lane M, marker proteins.