Abstract
Objective:
There are many concerns about the interactions of herbal products with conventional drugs, which are mostly used as multiple drug treatment approach. The present study was designed to evaluate the effect of long-term use of Ginkgo biloba extract (GK) on the absorption and tissue distribution of fluoxetine and venlafaxine.
Materials and Methods:
46 Wistar rats are utilized and allocated into 8 groups; 2 groups administered the vehicle and saved as control; 4 groups are treated with 100 and 200 mg/kg of GK extract for 30 days; 2 groups are treated with 40 mg/kg verapamil for 10 days. The liver, kidney, and brain distribution of fluoxetine and venlafaxine were evaluated after single oral doses using high performance liquid chromatographic method.
Results:
200 mg/kg GK increases fluoxetine concentrations in all studied organs, while GK 100 mg/kg increases venlafaxine levels in kidney tissue and not affected in the other two organs.
Conclusion:
Thirty days treatment with GK (100 mg/kg) increases kidney availability of venlafaxine, while 200 mg GK dose increases fluoxetine availability in the liver, kidney, and brain tissues after single oral doses.
KEY WORDS: Absorption, fluoxetine, Ginkgo biloba, tissue distribution, venlafaxine
INTRODUCTION
Absorption of drug molecules from oral dosage forms represents one of the important functions of the gastrointestinal tract (GIT); however, its barrier functions are also responsible for restricting absorption of many xenobiotics including drugs [1]. Availability of metabolizing enzyme systems and effective transporters, especially P-glycoprotein (P-gp), in the GIT epithelium protects many sensitive tissues and organs against invasion by many xenobiotics [2], in addition to the secretion of various metabolites into the GI lumen [3,4]. The safety and efficacy of many drugs depend on many variables involved in their interactions with the components of the biological system; such interactions may influence pharmacokinetic properties of introduced drugs, including their affinity for the available membrane transporters, with consequent changes in absorption, distribution, and elimination processes [5]. After absorption, distribution of drug molecules between tissues and organs depends on many variables; among them are regional blood flow, epithelial permeability, and protein binding. Moreover, the extent of transporter’s expression plays a major role in this respect, especially for specific selective ligands [6]. The barrier and transport functions of these membrane transporters can be negatively or positively influenced by many ligands, including natural herb preparations like G. biloba extract (GK) [7,8]. Although the influence of GK on the absorption and distribution of many drugs is not extensively evaluated, the overall capacity of small doses of this extract to modify the functions of drug transporters shows conflicting results [9,10]. Such type of interference, when occurs, may change the pharmacokinetic behavior of many therapeutic agents, which may predispose to fatal consequences, especially in those with low therapeutic index or those used for treatment of disorders in the central nervous system. The present study aims to evaluate the effects of long-term administration of GK on the absorption and tissue distribution of fluoxetine and venlafaxine after single oral doses in rats.
MATERIALS AND METHODS
A total of 46 adult male of Wistar rats (150-200 g) were used in the study. The animals were breaded in the Animal House, College of Pharmacy, University of Baghdad, and housed in the Animal House of the College of Medicine, University of Babylon. They were kept in a polypropylene cages at controlled temperature (25 ± 2°C), and were allowed to acclimatize for at least 1-week prior to the experiment. They had free access to commercial pellet diet and water ad libitum. The research protocol was approved by the Local Research Ethics Committee of the College of Pharmacy, University of Baghdad, and in accordance with International Requirements of the Experimental Animal Research. The rats were randomly allocated into 8 Groups, 6 rats in each group (except verapamil - treated group), and treated as follow [Figure 1]: Two groups were given 5% carboxymethylcellulose (CMC; Beijing Shouke Hongtai Chem Technol Co, China) (the vehicle) orally by oral gavage tube for 30 days; and then given a single oral doses of fluoxetine (20 mg/kg) or venlafaxine (20 mg/kg) (Hefei Joye Import Export Co., China) which served as control groups; 2 Groups were given GK (100 mg/kg/day) (premier-health, UK) suspended in 5% CMC orally by gavage tube for 30 days, and then given single doses of fluoxetine (20 mg/kg) or venlafaxine (20 mg/kg) orally, while other two groups of rats were treated similarly except for the GK dose, which was 200 mg/kg/day. In the last two groups (5 rats each), the rats were given 40 mg/kg/day of verapamil (Abbott, UK) orally by gavage tube for 10 days [11], and then given single doses of fluoxetine (20 mg/kg) or venlafaxine (20 mg/kg) orally. All animals were sacrificed after 5.5 and 2.0 h after administration of the fluoxetine [12] and venlafaxine doses [13], respectively. Blood samples were aspirated directly from the heart into polyethylene tube, and left to clot, centrifuged at 10000 rpm for 20 min. The resulted serum was kept frozen (−40°C) until the time of drugs analysis. Livers and both kidneys were quickly removed, and perfused with ice-cooled saline (4°C) to remove the blood. After decapitation, the whole brain was carefully removed, rinsed in saline at 4°C, and the arachnoid membrane was carefully removed. 500 mg of liver tissue and 300 mg of kidney and brain tissues were excised, and homogenized with Teflon-head homogenizer. The tissue homogenates were stored in deep freeze (−80°C) to be used for drugs analysis.
Figure 1.
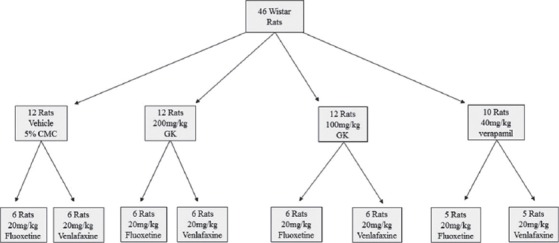
Flow chart of study design and animal groups
Determination of Serum Fluoxetine and Venlafaxine
A volume of 50 µl aliquot of serum was deproteinized with a 100 µl aliquot of acetonitrile. After vortex mixing and centrifugation (16,000 rpm, 10 min), 30 µl of the supernatant was injected directly into a reversed-phase (C18) high performance liquid chromatographic (HPLC) column.
Measurements of Fluoxetine and Venlafaxine Levels in Brain, Kidney, and Liver Tissues
The tissue homogenate of each organ was deproteinized with 400 µl of acetonitrile and 500 µl of methanol, centrifuged, filtered, and evaporated to dryness at 45°C using vacuum oven. The resulted dry material was reconstituted with 100 µl of the mobile phase, and centrifuged again. Then 30 µl of the obtained supernatant was directly injected into the HPLC (Knauer, Germany). The samples were not diluted before HPLC measurement. Unknown concentrations of fluoxetine and venlafaxine were interpolated from a respective standard curves prepared previously [14].
Statistical Analysis
All values, which represent the tissue/serum ratio of drug concentrations, were expressed as mean ± standard deviation; the results were statistically evaluated using unpaired Student’s t-test and one-way Analysis of Variance, supported by Bonferroni’s post hoc analysis. Values with P < 0.05 were considered significantly different. Analysis was performed using graph pad prism software for Windows, version 5.0 (Graph Pad Software, Inc., San Diego, CA).
RESULTS
Figure 2 shows that the effect of long-term administration of GK (200 mg/kg) on the distribution of fluoxetine in the liver tissue was significantly higher than that produced by the vehicle, verapamil (40 mg/kg) and GK (100 mg/kg), respectively. Meanwhile, there were no significant differences between the effects of verapamil and 100 mg/kg GK on the distribution of fluoxetine in the liver tissue (P > 0.05). Figure 3 shows that the effects of GK (200 mg/kg) and verapamil (40 mg/kg) on the distribution of fluoxetine in kidney tissue were significantly higher than that produced by the vehicle and 100 mg/kg GK, respectively, and no significant differences reported between the effects of GK (100 mg/kg) and the vehicle in this organ (P > 0.05). Moreover, Figure 4 shows that the effect of GK (200 mg/kg) on the distribution of fluoxetine in the brain tissue was significantly higher than that produced by the administration of the vehicle alone or 100 mg/kg GK. Meanwhile, the effect of verapamil was significantly higher than that reported in the vehicle-treated animals or 100 mg/kg GK. In Figure 5, no significant difference reported between the effect of GK (100 and 200 mg/kg) and verapamil on the distribution of venlafaxine in the liver tissue (P > 0.05). In addition, Figure 6 shows that the effects of GK (100 mg/kg) on the distribution of venlafaxine in the kidney tissue was significantly higher than that produced by either the vehicle alone, verapamil (40 mg/kg) or GK (200 mg/kg). Meanwhile, both verapamil (40 mg/kg) and GK (200 mg/kg) significantly decreased the extent of venlafaxine distribution, compared with that produced by the vehicle alone. There was no significant difference (P > 0.05) between the effect of verapamil (40 mg/kg) and GK (200 mg/kg) in this respect. Figure 7 shows that the effect of verapamil (40 mg/kg) on venlafaxine distribution in the brain tissue was significantly higher than that produced by either the vehicle alone or the two doses of GK. Meanwhile, there was no significant difference between the effects of the two doses of GK on the distribution of venlafaxine in brain tissue.
Figure 2.
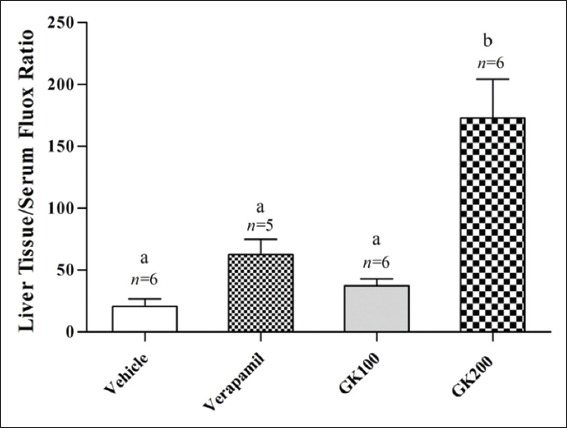
Effect of Ginkgo biloba extract (100 and 200 mg/kg) and verapamil (40 mg/kg) on the liver tissue/serum ratio of fluoxetine in rats challenged with single oral dose of fluoxetine (20 mg/kg); n: Number of animals; values with non-identical letters (a,b) are significantly different (P < 0.05)
Figure 3.
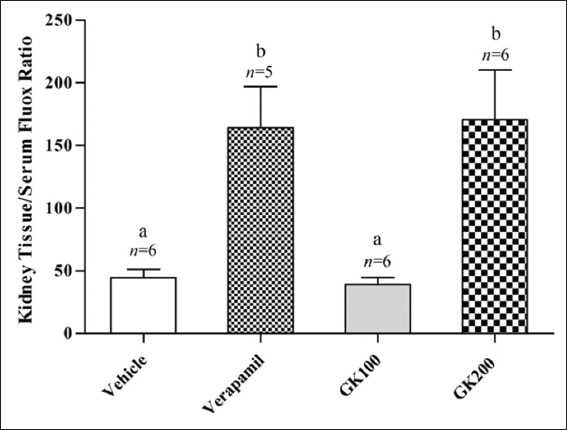
Effect of Ginkgo biloba extract (100 and 200 mg/kg) and verapamil (40 mg/kg) on the kidney tissue/serum ratio of fluoxetine in rats challenged with single oral dose of fluoxetine (20 mg/kg); n: Number of animals; values with non-identical letters (a,b) are significantly different (P < 0.05)
Figure 4.
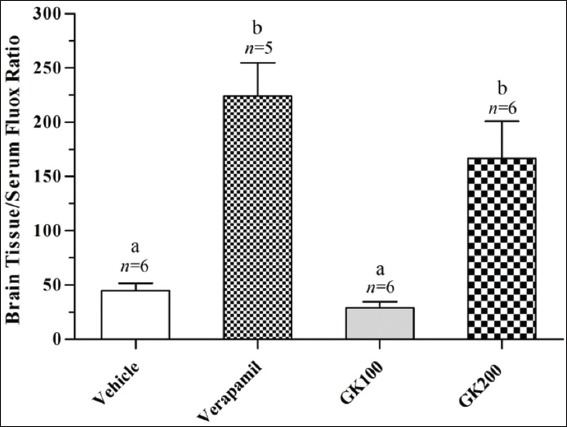
Effect of Ginkgo biloba extract (100 and 200 mg/kg) and verapamil (40 mg/kg) on the brain tissue/serum ratio of fluoxetine in rats challenged with single oral dose of fluoxetine (20 mg/kg); n: Number of animals; values with non-identical letters (a,b) are significantly different (P < 0.05)
Figure 5.
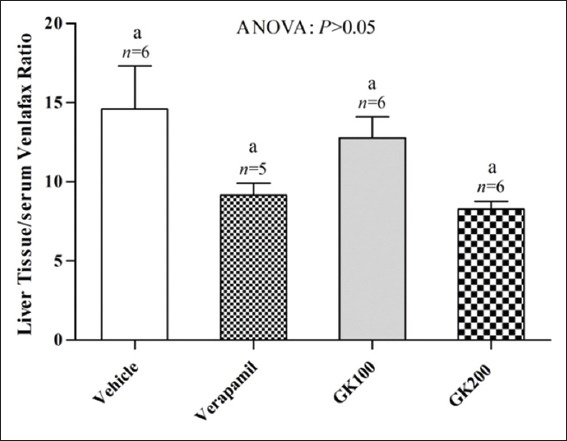
Effect of long-term administration of Ginkgo biloba extract (100 and 200 mg/kg) and verapamil (40 mg/kg) on the liver tissue/serum ratio of venlafaxine in rats challenged with single oral dose of venlafaxine (20 mg/kg); n: Number of animals; values with non-identical letters are significantly different (P < 0.05)
Figure 6.
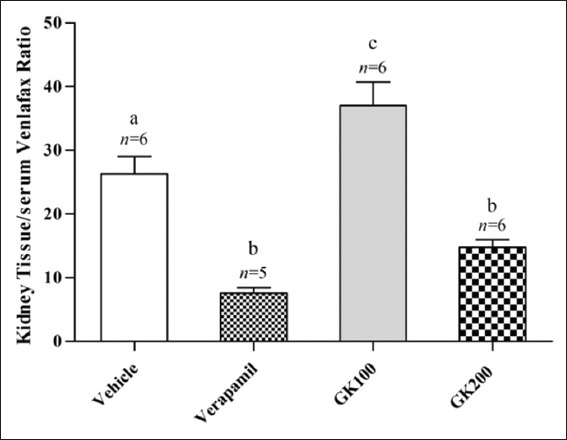
Effect of Ginkgo biloba extract (100 and 200 mg/kg) and verapamil (40 mg/kg) on the kidney tissue/serum ratio of venlafaxine in rats challenged with single oral dose of venlafaxine (20 mg/kg); n: Number of animals; values with non-identical letters (a-c) are significantly different (P < 0.05)
Figure 7.
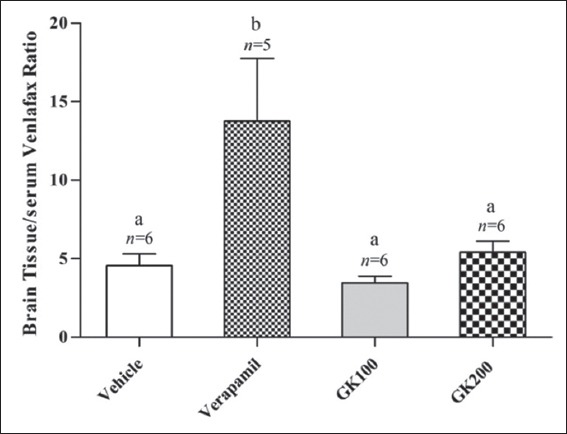
Effect of long-term administration of Ginkgo biloba extract (100 and 200 mg/kg) and verapamil (40 mg/kg) on the brain tissue/serum ratio of venlafaxine in rats challenged with single oral dose of venlafaxine (20 mg/kg); n: Number of animals; values with non-identical letters (a,b) are significantly different (P < 0.05)
DISCUSSION
Many animal studies, which investigate the expected interactions of GK with synthetic drugs, recognized the importance of justifying whether interference with tissue transporters by GK is relevant to be considered during clinical trials or not; particularly in drugs such as fluoxetine and venlafaxine, which are potential ligands for many affected transporters such as P-gp or others. The inhibition of transport activities in vitro can be extrapolated to the herb-drugs interaction potential in vivo, with effective high serum concentration, high tissue/serum fraction, and may be accompanied by exaggerated pharmacodynamic activity [15]. However, extracts obtained from G. biloba consist of many compounds, and this make the identification of a single effective constituent of these extracts and their plasma concentration a little bit difficult. Therefore, we compare the in vivo GK-drugs interaction potential in rats with that produced by verapamil, a simultaneous substrate for the expected drug transporters. In the present study, GK significantly increases liver tissue concentration of fluoxetine, while the tissue/serum ratio in brain and kidney are not significantly changed. Elevated hepatic level of fluoxetine may be attributed to the inhibitory effect of GK on P-gp in the liver tissue that increases its hepatic uptake. This finding was in tune with that reported by Hellum and Nilsen (2007), who indicated that GK is the most potent inhibitor of P-gp activity, among the tested herbs in their investigation [8]. Meanwhile, other finding contradicts the results of the present study. Li et al. (2009) found that GK increases P-gp gene expression in primary human hepatocytes, where the systemic availability of simvastatin is reduced secondarily to GK-mediated P-gp induction [16]. The reported data in the present study indicate that the low dose of G. biloba extract may have a mild inhibitory effect on the P-gp transporter activity. Moreover, increasing GK dose significantly increases in the tissue/serum ratio of fluoxetine in all the studied organs. These data indicate that GK may have a potent inhibitory effect on P-gp activity at high dose, which may lead to enhance fluoxetine uptake in all the studied organs. Other researchers studied the effect of acute and chronic use of GK on the P-gp function, without specific emphasis on the dose-response relationship in this respect. Yang et al. (2006) reported that GK decreases cyclosporine oral bioavailability in rats, due to increased expression of P-gp in the small intestine after long-term exposure [17]. This finding apparently contradicts the results obtained in our study. However, the effect of G. biloba might be similar to that reported for St. John’s Wort. It reflects inhibition at short-term (acute exposure) in vitro and in vivo, and induction in vivo, only after a long-term administration (repeated exposure) [9,18]. Similar finding was reported for the interaction of many pure polyphenols, some of them are constituents of GK, with the absorption and distribution of metformin (P-gp ligand), while atenolol (not P-gp ligand) was not affected [19]. In the present study, tissue/serum ratios of venlafaxine were increased significantly in the kidney tissue, while not significantly changed in the liver and brain tissues. Since venlafaxine is a known P-gp ligand, its elevated concentration in the kidney tissue might be due to the inhibitory effect of GK on this efflux transporter (in the apical regions of the vascular epithelium). This finding comes in tune with that reported by Bent (2008), who indicates that GK is the most potent inhibitor of P-gp activity among the natural products that are tested in his investigation [8]. In addition, we previously reported that 200 mg/kg of GK significantly increases venlafaxine concentrations in the serum [20], and associated with significant decrease in the tissue/serum ratio in the liver and kidney tissues, as shown in the present study. This can be explained on the bases that the elevated serum levels might be attributed to the potent inhibitory effect of GK on the intestinal P-gp, which leads to increased venlafaxine absorption. Although the present study demonstrates well the significant influence of GK on absorption and tissue distribution of fluoxetine and venlafaxine in rats, such activity may not be necessarily observed in human. This idea is in accordance with many reports in this respect, and human studies in this field are highly suggested [21,22]. The inhibitory effect of GK on the activity of P-gp, which expel out drug molecules, may enhance liver and kidney uptake of venlafaxine, and elevates its concentration significantly in the studied organs. The elevation in serum concentration of venlafaxine is higher than that reported in the liver and kidney tissues, and this may explain the significant decrease in tissue/serum ratio in these organs. Since drug transporters can play pivotal roles in drug handling within the biological system, many efforts during drug discovery and development process are directed to synthesize or isolate new chemical compounds, which interfere or not with P-gp, depending on the desired type of interaction. Accordingly, P-gp inhibitors are developed to improve penetration of drug molecules into neoplastic cells and optimize chemotherapy. Similarly, this approach is also followed to facilitate penetration of CNS-active drugs into the brain tissue to overcome the pharmacoresistance associated with many CNS disorders (e.g., Parkinson’s disease). Meanwhile, many chemicals that are P-gp ligands should be identified early during the stage of development. At this level, it is important to consider the suggested idea that expression of drug transporters and CYP enzymes might be functionally linked to limit oral drug bioavailability [23]. In the intestine most of the interactions that involve drug transporters, and responsible for the efflux of drugs, often result in poor absorption and low oral bioavailability. Since the majority of drugs are developed as oral dosage forms, the use of animal models to evaluate their interactions at that site have become critical tools, for assessing a drug’s potential in vivo absorption properties in the presence of expected modulators of membrane transporters.
CONCLUSION
30 days treatment with G. biloba extract (100 mg/kg) increases kidney availability of venlafaxine, while 200 mg dose increases fluoxetine availability in the liver, kidney, and brain tissues after single oral doses.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Terada T, Hira D. Intestinal and hepatic drug transporters: Pharmacokinetic, pathophysiological, and pharmacogenetic roles. J Gastroenterol. 2015;50:508–19. doi: 10.1007/s00535-015-1061-4. [DOI] [PubMed] [Google Scholar]
- 2.Laksitorini M, Prasasty VD, Kiptoo PK, Siahaan TJ. Pathways and progress in improving drug delivery through the intestinal mucosa and blood-brain barriers. Ther Deliv. 2014;5:1143–63. doi: 10.4155/tde.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50:161–78. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 4.Ma JD, Tsunoda SM, Bertino JS, Jr, Trivedi M, Beale KK, Nafziger AN. Evaluation of in vivo P-glycoprotein phenotyping probes: A need for validation. Clin Pharmacokinet. 2010;49:223–37. doi: 10.2165/11318000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Drozdzik M, Gröer C, Penski J, Lapczuk J, Ostrowski M, Lai Y, et al. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol Pharm. 2014;11:3547–55. doi: 10.1021/mp500330y. [DOI] [PubMed] [Google Scholar]
- 6.Chillistone S, Hardman J. Factors affecting drug absorption and distribution. Anaesth Intensive Care Med. 2011;12:151–5. [Google Scholar]
- 7.Abad MJ, Bedoya LM, Bermejo P. An update on drug interactions with the herbal medicine G. biloba. Curr Drug Metab. 2010;11:171–81. doi: 10.2174/138920010791110818. [DOI] [PubMed] [Google Scholar]
- 8.Hellum BH, Nilsen OG. In vitro inhibition of CYP3A4 metabolism and P-glycoprotein-mediated transport by trade herbal products. Basic Clin Pharmacol Toxicol. 2008;102:466–75. doi: 10.1111/j.1742-7843.2008.00227.x. [DOI] [PubMed] [Google Scholar]
- 9.Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006;78:2131–45. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Fan L, Tao GY, Wang G, Chen Y, Zhang W, He YJ, et al. Effects of G. biloba extract ingestion on the pharmacokinetics of talinolol in healthy Chinese volunteers. Ann Pharmacother. 2009;43:944–9. doi: 10.1345/aph.1L656. [DOI] [PubMed] [Google Scholar]
- 11.Kirchengast M. Preclinical considerations and results with the combination of verapamil and trandolapril: Blood pressure reduction and beyond. J Hypertens. 1997;15:S27–33. [PubMed] [Google Scholar]
- 12.Keller T, Cambon N, Genevray M, Crivelli F, Crivelli M, Dal BL, et al. Bioequivalence study of fluoxetine hydrochloride in healthy volunteers. Arzneimittelforschung. 2005;55:491–7. doi: 10.1055/s-0031-1296895. [DOI] [PubMed] [Google Scholar]
- 13.Goodnick PJ. Pharmacokinetic optimisation of therapy with newer antidepressants. Clin Pharmacokinet. 1994;27:307–30. doi: 10.2165/00003088-199427040-00005. [DOI] [PubMed] [Google Scholar]
- 14.Eswarudu MM, Anitha M, Gayathri N, Chaithanya T. A validated RP-HPLC method for the simultaneous estimation of fluoxetine hydrochloride and olanzapine in pharmaceutical dosage form. Int Res J Pharm. 2012;3:310–3. [Google Scholar]
- 15.Dresser MJ, Gray AT, Giacomini KM. Kinetic and selectivity differences between rodent, rabbit, and human organic cation transporters (OCT1) J Pharmacol Exp Ther. 2000;292:1146–52. [PubMed] [Google Scholar]
- 16.Li L, Stanton JD, Tolson AH, Luo Y, Wang H. Bioactive terpenoids and flavonoids from G. biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm Res. 2009;26:872–82. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CY, Chao PD, Hou YC, Tsai SY, Wen KC, Hsiu SL. Marked decrease of cyclosporin bioavailability caused by coadministration of ginkgo and onion in rats. Food Chem Toxicol. 2006;44:1572–8. doi: 10.1016/j.fct.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Dürr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, et al. St John's Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 19.Hussain SA, Jaccob AA. Effects of long-term use of polyphenols on the absorption and tissue distribution of orally administered metformin and atenolol in rats. J Intercult Ethnopharmacol. 2013;2:147–54. [Google Scholar]
- 20.Alzubaidi FA, Hussain SA. Effects of G. biloba extract on the oral bioavailability of fluoxetine and venlafaxine in rats. Am J Pharm Sci. 2015;3:7–12. doi: 10.5455/jice.20150628102732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song IS, Kong TY, Jeong HU, Kim EN, Kwon SS, Kang HE, et al. Evaluation of the transporter-mediated herb-drug interaction potential of DA-9801, a standardized dioscorea extract for diabetic neuropathy, in human in vitro and rat in vivo. BMC Complement Altern Med. 2014;14:251. doi: 10.1186/1472-6882-14-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji HY, Liu KH, Kong TY, Jeong HU, Choi SZ, Son M, et al. Evaluation of DA-9801, a new herbal drug for diabetic neuropathy, on metabolism-mediated interaction. Arch Pharm Res. 2013;36:1–5. doi: 10.1007/s12272-013-0014-9. [DOI] [PubMed] [Google Scholar]
- 23.Kivistö KT, Niemi M, Fromm MF. Functional interaction of intestinal CYP3A4 and P-glycoprotein. Fundam Clin Pharmacol. 2004;18:621–6. doi: 10.1111/j.1472-8206.2004.00291.x. [DOI] [PubMed] [Google Scholar]


