Fig. 2.
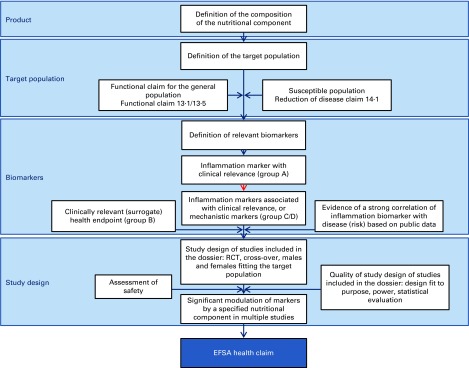
Schematic of topics to be addressed when building a dossier for a European Food Safety Authority (EFSA) health claim on control of chronic low-grade inflammation. The blue boxes indicate the main topics to be addressed; the white boxes state the actual content topics. Building a strong EFSA health claim dossier requires (1) a definition of the composition of the nutritional component including manufacturing procedures in scope and out of scope for the claim, (2) a clear definition of the target population, being the general population or a specific subpopulations at risk, including the defining parameters, (3) a definition of biomarkers measured to assess the health effects of the nutritional component, including a description of the proof of clinical relevance, or the clinical validity of the combination of inflammation biomarkers and related clinically relevant biomarkers for health benefit endpoints associated with the health claim, and (4) a full description of clinical study design for all studies included in the dossier, including statistical power analysis and safety evaluation. The red arrow indicates the primary hurdle for functional health claims in the area of chronic low-grade inflammation, which is the lack of (combinations of) inflammation biomarkers with established and therefore accepted clinical relevance. This is primarily the consequence of inflammatory responses being non-specific normal physiological responses to tissue damage, and discrimination between normal and abnormal levels or combinations has not been well established in relation to chronic low-grade inflammation. The description of the classification of clinical relevance of biomarkers (categories A–D) was adapted from Albers et al. ( 137 ). RCT, randomised controlled trial. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
