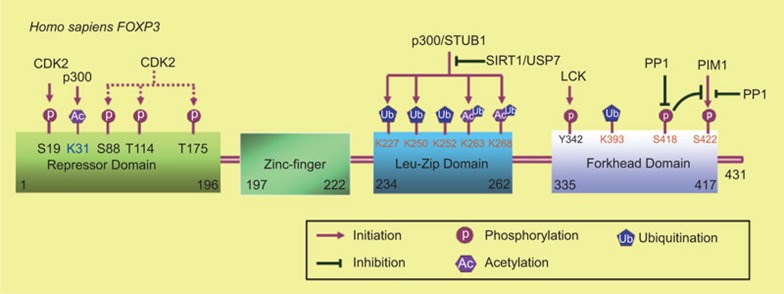
Figure 1.
Identified enzymes that are responsible for the post-translational modifications of FOXP3 and their corresponding modified residues in human FOXP3. Acetylation of FOXP3 at K263 and K268 by the acetyltransferase p300 stabilizes FOXP3 and promote its activity, while SIRT1 modulates the reciprocal modification. The E3 ligase STUB1 promotes the ubiquitination of FOXP3 at its leucine-zipper domain (K227, K250, K252, K263 and K268) and leads to its degradation, while the deubiquitinase USP7 prevents the ubiquitination process and stabilizes FOXP3. Acetylation and ubiquitination may compete for the same lysine residues in the leucine-zipper domain of FOXP3 to affect its stability and activity. FOXP3 is highly phosphorylated at Ser418 and dephosphorylation at the residue mediated by the phosphotase PP1 downregulates its repressive activity; The kinase PIM1 phosphorylates FOXP3 at Ser422 and negatively regulates FOXP3 activity, while the phosphatase PP1 works oppositely. Furthermore, phosphorylation at Ser418 prevents PIM1-mediated phosphorylation at Ser422. CDK2 phosphorylates FOXP3 at Ser88, Thr144 and Thr175 and promotes FOXP3 degradation. LCK phosphorylates FOXP3 at Tyr342 and enhances the repressive activity of FOXP3. CDK2, cyclin-dependent kinase 2; LCK, lymphocyte-specific protein kinase; PP1, protein phosphatase 1.