Table 1. Comparison between Na2CoP2O7 and CoOx.
| Catalyst | Na2CoP2O7 | CoOx (Co-Pi, β-CoOOH) | Refs |
|---|---|---|---|
| Electrochemical performance | |||
| Catalyst type | Powder (crystalline) | Electrodeposited thin film (amorphous) | 8 |
| Experimental overpotential value | 560 mV (1 mA cm−2) | 410 mV (1 mA cm−2) | 8 |
| pH (electrolyte composition ) | pH 7 (0.5 M phosphate) | pH 7 (0.1 M phosphate) | 8 |
| Tafel slope | Approximately 80∼90 mV dec−1 | 60 mV dec−1 | 8,30 |
| pH dependence | 67 mV | 64 mV | 47 |
| Buffer concentration dependence | Zeroth order dependence | Zeroth order dependence | 47 |
| Structural/chemical property during OER | |||
| EPR | 2+ or 2+/3+ mixture | 3+/4+ mixture | 30 |
| In-situ XANES | 2+ | ⩾3+ | 26 |
| HR-TEM at surface (EDX) | High crystallinity (Co: P=1:2, TEM) | Amorphous phase (Co: P=2∼3:1, SEM) | 8 |
| OER mechanism | |||
| Cobalt coordination (Pristine state) | 4 (Tetrahedral) | 6 (Octahedral) | 26,30 |
| Cobalt coordination (During OER) | 4↔5 | 5↔6 | 31 |
| OER active surface | (101) | 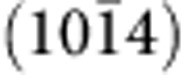 |
31 |
| Rate determining Step | OH*→O* | O*→OOH* (acid–base mechanism) | 31 |
| Theoretical overpotential value | 417 mV | 480 mV (ref) 437 mV (our work) | 31 |
EDX, scanning transmission electron microscopy; EPR, electron paramagnetic resonance; HR-TEM, high-resolution transmission electron microscopy; OER, oxygen evolution reaction; XANES, X-ray absorption near edge spectroscopy.
