Abstract
The present study investigated the clinicopathological characteristics of resected hepatitis B virus surface antigen (HBs-Ag)-negative, hepatitis C virus antibody (HCV-Ab)-negative hepatocellular carcinoma (NBNC HCC). The clinicopathological characteristics of 164 patients with NBNC HCC, 144 patients with HBs-Ag-positive HCC (HBV group) and 550 patients with HCV-Ab-positive HCC (HCV group) were compared. In the NBCN HCC group, 61 patients succumbed after 2 years. Subsequently, NBCN HCC patients were compared according to survival time (<2 years, 39 patients vs. ≥2 years, 64 patients) to identify prognostic factors. Finally, the clinicopathological characteristics of NBNC HCC were compared according to history of alcohol abuse/pathological results: Non-alcoholic steatohepatitis HCC (NASH group, 40 patients), alcohol abuse HCC (AL group, 80 patients) and other HCCs (non-NASH/non-AL group, 44 patients). Age, diabetes prevalence and body mass index were significantly higher for NBNC HCC compared with virus-related HCC. Among stage II cases, the prognosis was significantly better for the NBNC compared with that for the HCV group. A high α-fetoprotein level, poorly differentiated HCC and advanced liver fibrosis were independent risk factors for the prognosis of NBNC HCC. The proportion of female patients was significantly higher among NASH compared with AL HCC patients. The cumulative survival rates following surgery were similar in the NASH, AL and non-NASH/non-AL groups. NBNC HCC is considered to be a lifestyle disease, with better prognosis for stage II patients. The prognostic factors for NBNC HCC patients undergoing hepatectomy were similar to those with virus-related HCC and did not differ according to alcohol abuse history or pathological results.
Keywords: hepatitis B surface antigen, hepatitis C antibody, hepatocellular carcinoma, non-alcoholic steatohepatitis
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of mortality in Asian countries, including Japan, as well as in Africa and Europe. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are known to be important risk factors for the development of HCC (1,2). Carcinogenesis due to HBV and/or HCV infection has been decreasing in Japan, due to the reduction in the number of new viral infections. However, the number of HCC patients with neither HBV nor HCV infection (NBNC HCC) has been increasing annualy, currently accounting for 15% of all HCC cases in Japan (3). In NBNC HCC cases, carcinogenesis may be attributable to a history of alcohol abuse (AL HCC) or non-alcoholic steatohepatitis (NASH HCC), in which a progressively fatty liver leads to cirrhosis despite the absence of a history of alcohol abuse. Furthermore, carcinogenesis may occur in a minority of patients with normal background liver tissue and no history of alcohol abuse. In addition, cases positive for the hepatitis B core antibody (HBc-Ab), indicating a history of infection, may be classified as NBNC HCC if they are negative for the hepatitis B surface antigen (HBs-Ag). However, the criteria to determine whether these cases should be classified as NBNC HCC have not yet been clearly determined. The clinicopathological characteristics of NBNC HCC have recently attracted attention. The present study evaluated patients who underwent NBNC HCC resection at a single center, with the purpose of comparing the post-resection outcomes of NBNC HCC with those of virus-related HCC according to disease stage. In addition, we aimed to elucidate the factors contributing to poor prognosis in NBNC HCC, classify NBNC HCC according to etiology (NASH, AL and non-NASH/non-AL) and investigate its characteristics and outcomes.
Patients and methods
Patients
Of the patients diagnosed with HCC between March, 2001 and the end of March, 2013, 164 were negative for HBs-Ag and HCV-Ab according to preoperative blood tests and underwent HCC resection (NBNC group); 144 were positive for HBs-Ag and underwent HCC resection (HBV group); and 550 were positive for HCV-Ag and underwent HCC resection (HCV group). Patients who were positive for HBc-Ab and those positive for both HBs-Ag and HCV-Ab were excluded from the analysis. The diagnosis of HCC was based on abdominal contrast-enhanced computed tomography, magnetic resonance imaging, or ultrasonography findings. All the studies were approved by the Committee of Medical Ethics of Meiwa Hospital (Nishinomiya, Japan).
Study design
Study 1
The NBNC, HBV and HCV groups were compared with regard to age, maximum tumor size, tumor number, disease stage (Liver Cancer Study Group of Japan) (4), albumin level, total bilirubin level, aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, white blood cell (WBC) count, platelet count, prothrombin (PT) activity level, α-fetoprotein (AFP) level, des-γ-carboxyprothrombin (DCP) level, indocyanine green retention rate at 15 min (ICGR15), presence or absence of diabetes, Child-Pugh classification and body mass index (BMI). Patients ‘with diabetes’ were defined as those self-administering insulin and those receiving oral antidiabetic drugs. The post-resection outcomes were compared according to disease stage. The Kruskal-Wallis and χ2 tests were used to compare background factors among the groups. The Kaplan-Meier method and the log-rank test were used to compare outcomes.
Study 2
Sixty-one patients with NBNC HCC succumbed after 2 years. The remaining patients were divided into two groups: 39 patients with a survival time of <2 years (short survival; SS group) and 64 patients with a survival time of ≥2 years (long survival; LS group). The tumor and patient characteristics were compared between the two groups, and a multivariate analysis was performed with prognostic factors achieving significance (P<0.05) in the univariate analysis. The Mann-Whitney U test and the χ2 test were used for comparisons between the two groups, and multivariate logistic regression was used for the multivariate analysis.
Study 3
Patients with NBNC HCC were divided into three groups: 40 patients with pathologically diagnosed NASH (NASH group), 80 patients with a history of alcohol abuse (>20 g per day; AL group), and 44 patients without pathologically diagnosed NASH or history of alcohol abuse (non-NASH/non-AL group). The tumor and patient characteristics were compared among the three groups, and the post-resection outcomes were analyzed. The Kruskal-Wallis and χ2 tests were used for comparing background characteristics, whereas the Kaplan-Meier method and the log-rank test were used to evaluate outcomes. For each statistical test, P<0.05 was considered to indicate statistically significant differences. The statistical software used was JMP 9.0.2 (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Study 1
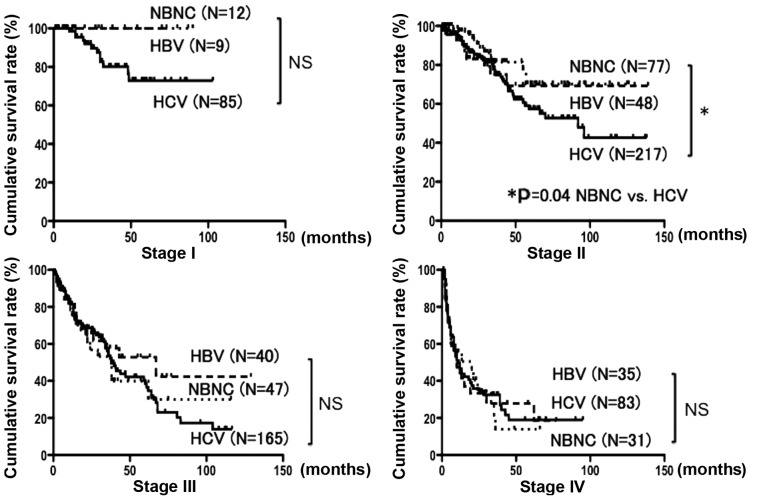
The patient characteristics according to HCC etiology are summarized in Table I. The average age in the NBNC group was the highest (70.3±7.8 years) and that in the HBV group the lowest (60.3±10.8 years). Tumor size was significantly larger in the NBNC (4.7±3.7 cm) and HBV (4.5±3.7 cm) groups compared with that in the HCV (3.2±2.2 cm) group. The albumin, WBC and platelet count were significantly increased, and AST and ALT levels were significantly decreased in the NBNC and HBV groups compared with those in the HCV group. The bilirubin level was significantly decreased and PT activity was significantly increased in the NBNC group compared with those in the HBV and HCV groups. ICGR15 was significantly decreased in the NBNC group compared with that in the HCV group, and the Child Pugh class A was significantly increased in the NBNC group compared with that in the HBV and HCV groups. The prevalence of diabetes was significantly higher in the NBNC group compared with that in the HBV and HCV groups. BMI was significantly higher in the NBNC group compared with that in the HBV and HCV groups (Table I). For the entire series, the cumulative survival rates at 1, 3 and 5 years were 85.0, 63.6 and 54.0%, respectively, in the NBNC group; 75.6, 56.5 and 53.0%, respectively, in the HBV group; and 83.5, 63.1 and 47.4%, respectively, in the HCV group. These differences were not significantly different (Fig. 1). On comparison according to disease stage, the cumulative survival rates for stage II disease at 1, 3 and 5 years were 97.2, 81.4 and 70.1%, respectively, in the NBNC group and 93.9, 75.8 and 57.3%, respectively, in the HCV group. Thus, the NBNC group exhibited a significantly more favorable prognosis (P=0.04). No significant differences were observed for stages I, III and IV (Fig. 2).
Table I.
Patient characteristics according to hepatocellular carcinoma etiology.
| Characteristics | NBNC (n=164) | HBV (n=144) | HCV (n=550) |
|---|---|---|---|
| Age (years)a | 70.3±7.8b,c | 60.3±10.8c | 69.4±7.6 |
| Gender (male/female) | 149/32c | 111/33 | 401/149 |
| Maximum tumor diameter (cm)a | 4.7±3.7c | 4.5±3.7c | 3.2±2.2 |
| Tumors (n)a | 1.9±2.8 | 2.0±1.6 | 2.0±2.1 |
| Disease stagea | 2.2±0.6 | 2.3±0.6c | 2.1±0.7 |
| Albumin (g/dl)a | 4.0±0.5c | 3.9±0.5c | 3.7±0.4 |
| Total bilirubin (mg/dl)a | 0.7±0.3b,c | 0.8±0.4 | 0.8±0.4 |
| AST (IU/l)a | 41±36c | 46±32c | 58±34 |
| ALT (IU/l)a | 36±36c | 39±32c | 52±37 |
| White blood cell count (/µl)a | 5,586±1,817c | 5,325±1,956c | 4,593±1,685 |
| Platelet count (x104/µl)a | 18.4±9.9c | 16.0±8.6c | 13.1±6.9 |
| Prothrombin activity (%)a | 87±13b,c | 79±13 | 82±16 |
| α-fetoprotein (ng/ml)a | 14,072±110,966c | 17,559±122,993c | 2,406±18,702 |
| DCP (mAU/ml)a | 11,827±74,826b,c | 6,726±45,768 | 1,707±8,522 |
| ICGR15 (%)a | 15±11c | 16±11 | 21±14 |
| Diabetes (+/-) | 87/94 (48%)b,c | 33/111 (23%) | 151/399 (27%) |
| Child-Pugh Classification (A/B) | 166/15b,c | 116/28 | 425/125 |
| BMI (kg/m2) | 23.9±3.8b,c | 22.7±3.8 | 22.5±3.1 |
Values are presented as means ± standard deviation.
P<0.05 vs. HBV.
P<0.05 vs. HCV. Disease stage represents the tumor-node-metastasis stage according to the Liver Cancer Study Group of Japan. NBNC, hepatitis B surface antigen-negative and hepatitis C virus antibody-negative patients; HBV, hepatitis B surface antigen-positive patients; HCV, hepatitis C virus antibody-positive patients; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DCP, des-γ-carboxyprothrombin; ICGR15, indocyanine green retention rate at 15 min; BMI, body mass index.
Figure 1.
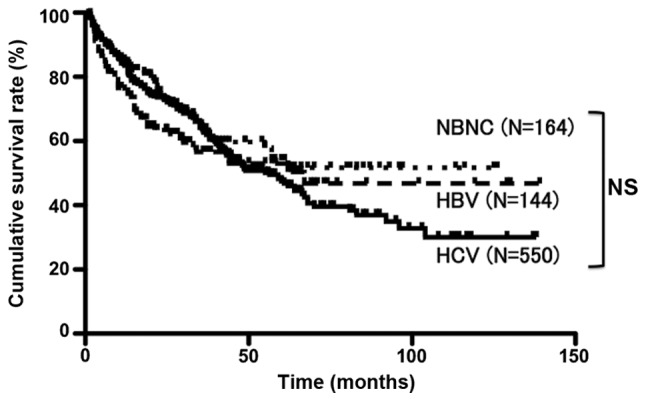
The cumulative survival rates of all resection cases over 5 years in the NBNC, HBV and HCV groups were 54, 53 and 47%, respectively; the differences were non-significant (NS). Continuous line, HCV; short dotted line, NBNC; and long dotted line, HBV. NBNC, hepatitis B surface antigen (HBs)-negative and HCV antibody-negative patients; HBV, hepatitis B surface antigen-positive patients; HCV, hepatitis C virus antibody-positive patients.
Figure 2.
On comparison according to disease stage, the prognosis in the NBNC group was significantly superior to that in the HCV group for stage II disease (P=0.04). The prognosis did not differ significantly between groups for stage I, III and IV disease. Continuous line, HCV; short dotted line, NBNC; and long dotted line, HBV. NBNC, hepatitis B surface antigen-negative and hepatitis C virus antibody-negative patients; HBV, hepatitis B surface antigen-positive patients; HCV, hepatitis C virus antibody-positive patients; NS, non-significant.
Study 2
The results of the univariate analysis for the SS and LS groups are presented in Table II. The groups differed significantly with regard to tumor size, tumor number, albumin, total bilirubin, DCP, AFP and AST levels, PT activity, ICGR15, Child-Pugh classification, tumor differentiation, degree of liver fibrosis and presence or absence of portal vein tumor thrombus (Table II). In the multivariate analysis of these factors, high AFP level, poorly differentiated HCC and advanced liver fibrosis were found to be independent risk factors (Table III).
Table II.
Univariate analysis of factors affecting prognosis in patients with NBNC hepatocellular carcinoma.
| Characteristics | SS group (n=39) | LS group (n=64) | P-value |
|---|---|---|---|
| Age (years)a | 69±8 | 69±7 | 0.47 |
| Gender (male/female) | 34/5 | 52/12 | 0.43 |
| Maximum tumor diameter (cm)a | 7.2±5.8 | 4.2±3.0 | 0.005 |
| Tumors (n)a | 2.5±2.5 | 1.5±1.1 | 0.01 |
| Albumin (g/dl)a | 3.8±0.5 | 4.2±0.4 | 0.01 |
| Total bilirubin (mg/dl)a | 0.8±0.4 | 0.7±0.3 | 0.01 |
| AST (IU/l)a | 58±52 | 34±22 | 0.001 |
| ALT (IU/l)a | 42±44 | 36±40 | 0.50 |
| White blood cell count (/µl)a | 5,397±2,054 | 5,870±1,684 | 0.06 |
| Platelet count (x104/µl)a | 19.3±7.4 | 18.5±7.2 | 0.69 |
| Prothrombin activity (%)a | 85.5±12.8 | 90.7±11.9 | 0.04 |
| α-fetoprotein (ng/ml)a | 64,222±236,188 | 201±847 | 0.0001 |
| DCP (mAU/ml)a | 54,224±162,995 | 1,677±5,669 | 0.02 |
| ICGR15 (%)a | 18.1±10.1 | 12.5±7.9 | 0.003 |
| Diabetes (+/-) | 16/23 | 33/31 | 0.29 |
| Child-Pugh Classification (A/B,C) | 32/7 | 61/3 | 0.02 |
| Pathological differentiation | |||
| (poor or moderate/high) | 15/24 | 1/63 | 0.0001 |
| Fibrosis stagea | 2.9±0.9 | 2.1±1.2 | 0.003 |
| BMI (kg/m2) | 23.4±3.5 | 24.1±3.6 | 0.61 |
| Tumor thrombus in portal vein (+/-) | 18/11 | 7/57 | 0.0001 |
Values are presented as means ± standard deviation. Fibrosis stage was determined according to the New Inuyama Classification. NBNC, hepatitis B surface antigen-negative and hepatitis C virus antibody-negative; SS, short survival (patients who succumbed to the disease within 2 years); LS, long survival (patients who survived for >2 years); AST, aspartate aminotransferase; ALT, alanine aminotransferase; DCP, des-γ-carboxyprothrombin; ICGR15, indocyanine green retention rate at 15 min; BMI, body mass index.
Table III.
Multivariate analysis of factors affecting prognosis in patients with NBNC hepatocellular carcinoma.
| Characteristics | P-value | Hazard ratio | 95% confidence interval |
|---|---|---|---|
| Maximum tumor diameter (cm) | 0.86 | 1.02 | −0.24–0.28 |
| Tumors (n) | 0.97 | 1.01 | −0.38–0.41 |
| Albumin (g/dl) | 0.22 | 0.34 | −0.29–0.66 |
| Total bilirubin (mg/dl) | 0.26 | 2.81 | −0.74–3.14 |
| AST (IU/l) | 0.34 | 1.01 | −0.01–0.04 |
| α-fetoprotein (ng/ml) | 0.04 | 1.89 | 0.001–1.33 |
| DCP (mAU/ml) | 0.10 | 2.13 | −0.15–1.75 |
| ICGR15 (%) | 0.55 | 1.02 | −0.05–0.09 |
| Child-Pugh Classification (A/B,C) | 0.88 | 0.81 | −3.14–2.53 |
| Pathological differentiation | |||
| (poor or moderate/high) | 0.003 | 18.70 | 0.89–6.01 |
| Fibrosis stage | 0.004 | 2.08 | 0.03–1.54 |
| Tumor thrombus in portal vein (+/-) | 0.26 | 2.50 | −0.72–2.57 |
Fibrosis stage was determined according to the New Inuyama Classification. NBNC, hepatitis B surface antigen-negative and hepatitis C virus antibody-negative; AST, aspartate aminotransferase; DCP, des-γ-carboxyprothrombin; ICGR15, indocyanine green retention rate at 15 min.
Study 3
The patient characteristics in the NASH, AL and non-NASH/non-AL groups are presented in Table IV. The proportion of female patients was significantly higher in the NASH group and the proportion of male patients was significantly higher in the AL group. There were no significant differences in the other background data (Table IV). The cumulative survival rates following surgery at 1, 3 and 5 years were 83.0, 62.2 and 54.5%, respectively, for the NASH group; 87.0, 69.7 and 56.3%, respectively, for the AL group; and 80.8, 42.0 and 33.6%, respectively, for the non-NASH/non-AL group. These differences were not statistically significant (Fig. 3).
Table IV.
Patient characteristics of NBNC hepatocellular carcinoma according to underlying liver background.
| Characteristics | NASH (n=40) | AL (n=80) | Non-NASH/non-AL (n=44) |
|---|---|---|---|
| Age (years)a | 71.6±8.1 | 68.9±7.3 | 71.3±8.1 |
| Gender (male/female) | 29/11b | 76/4c | 31/13 |
| Maximum tumor diameter (cm)a | 5.0±3.1 | 4.3±3.1 | 5.2±4.7 |
| Tumors (n)a | 2.3±2.1 | 1.7±1.4 | 1.7±1.7 |
| Disease stagea | 2.6±0.8 | 2.3±0.8 | 2.6±0.8 |
| Albumin (g/dl)a | 3.9±0.7 | 4.0±0.4 | 4.0±0.4 |
| Total bilirubin (mg/dl)a | 0.6±0.2 | 0.7±0.4 | 0.8±0.3 |
| AST (IU/l)a | 42±44 | 34±34 | 51±37 |
| ALT (IU/l)a | 33±30 | 35±36 | 45±48 |
| White blood cell count (/µl)a | 5,455±1,568 | 5,910±1,887 | 5,439±1,967 |
| Platelet count (x104/µl)a | 16.9±7.0 | 21.5±11.0 | 17.6±7.2 |
| Prothrombin activity (%)a | 84±16 | 90±14 | 87±12 |
| α-fetoprotein (ng/ml)a | 8,884±44,271 | 65±40,398 | 43,646±222,264 |
| DCP (mAU/ml)a | 2,373±5,235 | 1,103±87,819 | 17,558±88,206 |
| ICGR15 (%)a | 17.2±13.3 | 13.1±11.5 | 14.7±11.4 |
| Diabetes (+/-) | 15/25 | 41/39 | 21/23 |
| Child-Pugh Classification (A/B,C) | 35/5 | 74/6 | 42/2 |
| Fibrosis stagea | 2.3±1.3 | 2.2±1.1 | 2.4±1.3 |
| BMI (kg/m2) | 23.8±3.6 | 24.6±3.9 | 23.9±4.2 |
Disease stage represents the tumor-node-metastasis stage according to the Liver Cancer Study Group of Japan. Fibrosis stage was determined according to the New Inuyama Classification.
Values are presented as means ± standard deviation.
P<0.05 vs. AL.
P<0.05 vs. non-NASH/non-AL. NBNC, hepatitis B surface antigen-negative and hepatitis C virus antibody-negative; NASH, HCC patients with non-alcoholic steatohepatitis; AL, HCC patients consuming >20 g alcohol daily; AST, aspartate aminotransferase, ALT, alanine aminotransferase; DCP, des-γ-carboxyprothrombin; ICGR15, indocyanine green retention rate at 15 min; BMI, body mass index; HCC, hepatocellular carcinoma.
Figure 3.
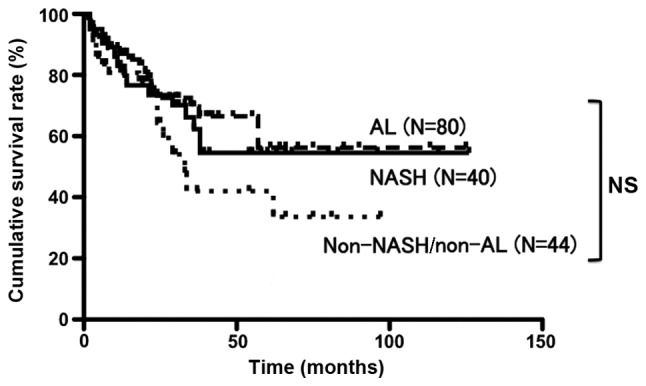
Post-surgical cumulative survival rates over 5 years for the NASH, AL and non-NASH/non-AL groups were 54.5, 56.3 and 33.6%, respectively. The prognosis was not significantly different. Continuous line, NASH; short dotted line, non-NASH/non-AL; and long dotted line, AL. NASH, HCC patients with non-alcoholic steatohepatitis; AL, HCC patients who consumed >20 g alcohol daily; non-NASH/non-AL, HCC patients without NASH or AL; NS, non-significant.
Discussion
The incidence of virus-related HCC has recently exhibited a decreasing trend due to the reduction in hepatitis virus transmission during blood transfusions and significant advances in antiviral therapy. However, the incidence of NBNC HCC has been increasing in Japan (2); consequently, the rate of resected NBNC HCCs in Meiwa Hospital has also been increasing. Between 2001 and 2003, the percentage of cases was 14% (NBNC HCC/virus-related HCC, 23/139); however this percentage increased to 22% (61/216) between 2010 and 2012, prompting the investigation of the clinicopathological characteristics of the NBNC HCC cases surgically treated at Meiwa Hospital.
The analysis of the characteristics of NBNC HCC indicated that the mean age, rate of complications associated with diabetes and BMI were significantly higher in these cases compared with cases of virus-related HCC, in agreement with previous reports (5–12). Patients with NBNC HCC exhibited a good liver function, although a number of patients presented with a large tumor size (6), possibly as they were not examined periodically due to the absence of hepatitis virus infection.
Comparisons according to disease stage revealed no significant difference in stage I cases between the NBNC, HBV and HCV groups, possibly due to the small sample size, although early-stage carcinoma (stages I and II) tended to exhibit a favorable prognosis. This may be explained by the fact that repetitive therapy was possible, as these patients exhibited a good liver function reserve compared with those with virus-related HCC (13–15). However, the prognosis was not significantly different between patients with NBNC HCC and those with virus-related HCC for stage III and IV cases.
The NBNC HCC prognostic factors in the present study included a high AFP value, poorly differentiated HCC and high degree of liver fibrosis. Distance from the resection margin, tumor multiplicity, the presence or absence of diabetes (12,16) and HBV DNA expression (17–20) are also reported to be factors associated with NBNC HCC prognosis. Thus, the prognostic factors for NBNC HCC identified in the present study are similar to those for virus-related HCC (21,22).
Furthermore, when patient characteristics were compared, it was revealed that the NASH group included a lower proportion of male patients compared with the AL and non-NASH/non-AL groups, and had a similar liver function reserve compared with the AL and non-NASH/non-AL groups. Clinically, patients with a history of alcohol abuse may be easily identified; however, it is more difficult to screen patients with NASH at high risk of carcinogenesis. In the present study, the mean BMI was 23.8 kg/m2, the platelet count was 16.9×104/µl, the AST and ALT levels were 42 and 33 IU/l, respectively, and the rate of diabetes complications was 38% (15/40) in the NASH group. Collectively, these findings suggest that diagnostic imaging should be considered in cases of abnormal AST or ALT levels.
The prognosis was not significantly different between the NASH, AL and non-NASH/non-AL groups. A history of alcohol abuse has been reported to increase the rate of HCC recurrence, as it promotes cirrhosis (23). However, in the present study, patients with a history of alcohol abuse did not have a worse prognosis; guidance was provided following surgery to help them quit, which may have improved their prognosis. The present study has helped elucidate the characteristics of NBNC HCC; however, it is necessary to establish a screening system for patients at high risk of hepatic carcinogenesis. A study including a large number of patients is required to identify high-risk groups and evaluate their treatment outcomes.
References
- 1.Ikeda K, Saitoh S, Koida I, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology. 1993;18:47–53. doi: 10.1002/hep.1840180109. [DOI] [PubMed] [Google Scholar]
- 2.Umemura T, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol Res. 2007;37(Suppl 2):S95–S100. doi: 10.1111/j.1872-034X.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagaoki Y, Hyogo H, Aikata H, et al. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42:368–375. doi: 10.1111/j.1872-034X.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 4.Liver Cancer Study Group of Japan, corp-author. General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 2nd English Edition. Kanehara & Co., Ltd.; Tokyo: 2010. p. 110. [Google Scholar]
- 5.Abe H, Yoshizawa K, Kitahara T, Aizawa R, Matsuoka M, Aizawa Y. Etiology of non-B non-C hepatocellular carcinoma in the eastern district of Tokyo. J Gastroenterol. 2008;43:967–974. doi: 10.1007/s00535-008-2264-8. [DOI] [PubMed] [Google Scholar]
- 6.Takamatsu S, Noguchi N, Kudoh A, et al. Influence of risk factors for metabolic syndrome and non-alcoholic fatty liver disease on the progression and prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:609–614. [PubMed] [Google Scholar]
- 7.Kusakabe A, Tanaka Y, Orito E, et al. A weak association between occult HBV infection and non-B non-C hepatocellular carcinoma in Japan. J Gastroenterol. 2007;42:298–305. doi: 10.1007/s00535-006-1999-3. [DOI] [PubMed] [Google Scholar]
- 8.Honda T, Miyaaki H, Ichikawa T, et al. Clinical characteristics of hepatocellular carcinoma in elderly patients. Oncol Lett. 2011;2:851–854. doi: 10.3892/ol.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Qin LX, Gong X, et al. Hepatitis B virus surface antigen-negative and hepatitis C virus antibody-negative hepatocellular carcinoma: clinical characteristics, outcome and risk factors for early and late intrahepatic recurrence after resection. Cancer. 2013;119:126–135. doi: 10.1002/cncr.27697. [DOI] [PubMed] [Google Scholar]
- 10.Kaneda K, Kubo S, Tanaka H, et al. Features and outcome after liver resection for non-B non-C hepatocellular carcinoma. Hepatogastroenterology. 2012;59:1889–1892. doi: 10.5754/hge10778. [DOI] [PubMed] [Google Scholar]
- 11.Kim SK, Marusawa H, Eso Y, et al. Clinical characteristics of non-B non-C hepatocellular carcinoma: a single-center retrospective study. Digestion. 2011;84(Suppl 1):43–49. doi: 10.1159/000333212. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura Y, Ikeda K, Arase Y, et al. Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol. 2008;23:1739–1746. doi: 10.1111/j.1440-1746.2008.05436.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka N, Tanaka T, Tanaka W, et al. Correlation of hepatitis virus serologic status with clinicopathologic features in patients undergoing hepatectomy for hepatocellular carcinoma. Cancer. 1997;79:1509–1515. doi: 10.1002/(SICI)1097-0142(19970415)79:8<1509::AID-CNCR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Yokoi Y, Suzuki S, Baba S, Inaba K, Konno H, Nakamura S. Clinicopathological features of hepatocellular carcinomas (HCCs) arising in patients without chronic viral infection or alcohol abuse: a retrospective study of patients undergoing hepatic resection. J Gastroenterol. 2005;40:274–282. doi: 10.1007/s00535-004-1536-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaibori M, Ishizaki M, Matsui K, Kwon AH. Clinicopathologic characteristics of patients with non-B non-C hepatitis virus hepatocellular carcinoma after hepatectomy. Am J Surg. 2012;204:300–307. doi: 10.1016/j.amjsurg.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Shinkawa H, Uenishi T, Takemura S, et al. Risk factors for postoperative recurrence of non-B non-C hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2010;17:291–295. doi: 10.1007/s00534-009-0186-3. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa H, Osaki Y, Arimoto A, Kita R, Kimura T. Relation between antibody to hepatitis B core antigen and survival after curative therapy for non-B non-C hepatocellular carcinoma. Anticancer Res. 2013;33:2211–2219. [PubMed] [Google Scholar]
- 18.Shi Y, Wu YH, Wu W, Zhang WJ, Yang J, Chen Z. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int. 2012;32:231–240. doi: 10.1111/j.1478-3231.2011.02481.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Kobayashi M, Someya T, et al. Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study. J Viral Hepat. 2009;16:437–443. doi: 10.1111/j.1365-2893.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakai T, Shiraishi O, Kawabe T, Ota H, Nagano H, Shiozaki H. Significance of HBV DNA in the hepatic parenchyma from patients with non-B, non-C hepatocellular carcinoma. World J Surg. 2006;30:1338–1343. doi: 10.1007/s00268-005-0318-0. [DOI] [PubMed] [Google Scholar]
- 21.Cescon M, Cucchetti A, Grazi GL, et al. Role of hepatitis B virus infection in the prognosis after hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a Western dual-center experience. Arch Surg. 2009;144:906–913. doi: 10.1001/archsurg.2009.99. [DOI] [PubMed] [Google Scholar]
- 22.Hanazaki K, Kajikawa S, Koide N, Adachi W, Amano J. Prognostic factors after hepatic resection for hepatocellular carcinoma with hepatitis C viral infection: univariate and multivariate analysis. Am J Gastroenterol. 2001;96:1243–1250. doi: 10.1111/j.1572-0241.2001.03634.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi Y, Horie Y, Kajihara M, et al. Hepatocellular carcinoma in heavy drinkers with negative markers for viral hepatitis. Hepatol Res. 2004;28:177–183. doi: 10.1016/j.hepres.2003.11.009. [DOI] [PubMed] [Google Scholar]