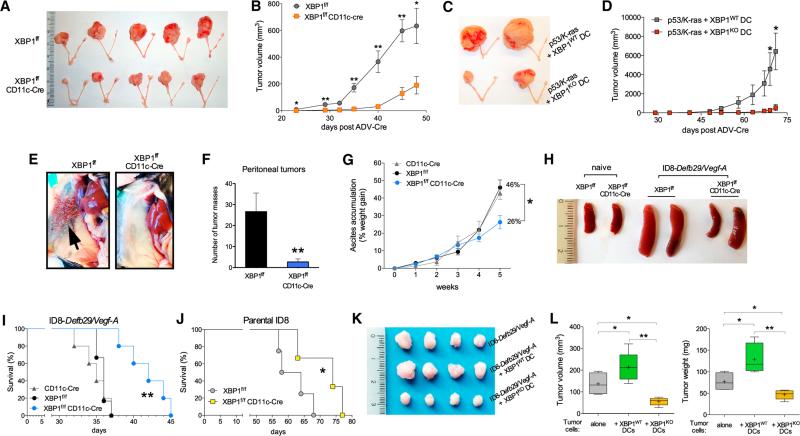
Figure 2. OvCa Progression in Hosts Lacking XBP1 in DCs.
(A) p53/K-ras-driven ovarian tumors were generated in hosts reconstituted with BM from either XBP1f/f (top) or XBP1f/f CD11c-Cre (bottom) donor mice. p53 deletion and oncogenic KRAS expression were induced via intrabursal injection of Cre-expressing adenovirus (ADV-Cre), as described in the Supplemental Experimental Procedures, and primary tumors were resected 7 weeks later. (B) Growth kinetics of p53/K-ras-driven tumors shown in (A). (C and D) Mice bearing primordial p53/K-ras-driven tumors for 28 days were injected i.p. with WT or XBP1-deficient (KO) BMDCs, and tumor growth was monitored over time until day 71. (E–H) Mice of the indicated genotypes were implanted with ID8-Defb29-VegfA OvCa cells via i.p. injection. (E and F) Peritoneal metastases evaluated 3–4 weeks after tumor implantation (n = 3 per group). (G) Malignant ascites generation in tumor-bearing mice expressed as percent weight gain due to progressive accumulation of peritoneal fluid. (H) Reduced splenomegaly in tumor-bearing mice deficient for XBP1 in CD11c+ DCs. (I and J) Survival in mice bearing aggressive ID8-Defb29-VegfA (I) or parental ID8 (J) tumors. Data are representative of at least two independent experiments with similar results using four to six mice per group. (K and L) ID8-Defb29-VegfA cancer cells were implanted in the flank alone or in combination with WT or XBP1-deficient (KO) BMDCs. Tumors were resected and measured 40 days later. *p < 0.05, **p < 0.001. Error bars represent SEM.