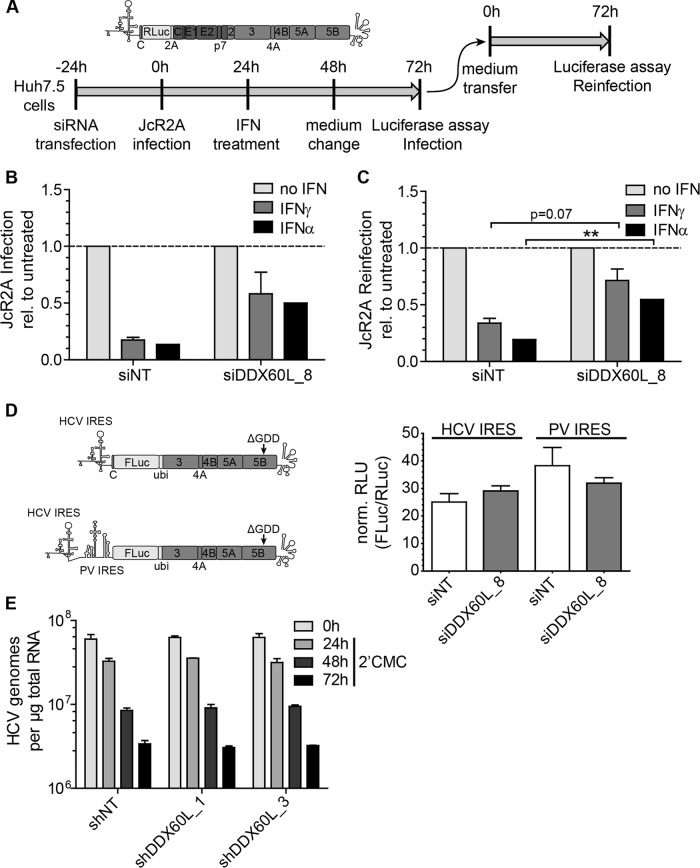
FIG 10.
Impact of DDX60L on HCVcc infection, IRES-dependent translation, and stability of HCV RNA. (A) Experimental strategy to determine the impact of DDX60L knockdown on replication and assembly and release of full-length HCV reporter virus JcR2A, schematically shown at the top. Huh7.5 cells were transfected with siRNAs 24 h before being infected with cell culture-derived JcR2A reporter virus at an MOI of 1 TCID50/cell. Twenty-four hours later, cells were treated with 0.3 ng/ml of IFN-γ or 5 IU/ml of IFN-α. Forty-eight hours after infection, the medium was replaced by interferon-free medium. Seventy-two hours after infection, cells were harvested and analyzed for luciferase activity to assess the contribution of DDX60L to the IFN response against HCV infection. Supernatants were transferred to naive Huh7.5 cells for quantification of HCV particle production (“reinfection”). Luciferase activity in the reinfection experiment was used as a correlate for titers of infectious virus. (B) JcR2A infection is rescued from IFN treatment by DDX60L knockdown. Huh7.5 cells transfected with the indicated siRNA and infected with JcR2A reporter virus were treated with IFN-γ or IFN-α and analyzed for Renilla luciferase activity as indicated in panel A. Luciferase values were normalized to the respective untreated controls to determine rescue of HCV infection from the IFN response by DDX60L knockdown. Shown are mean values with SDs (n = 2). (C) JcR2A particle production is rescued from IFN treatment by DDX60L knockdown. Huh7.5 cells were infected with supernatants from the samples in panel B. Seventy-two hours after reinfection, cell lysates were subjected to Renilla luciferase assay to analyze the rescue of HCV production from the IFN response upon silencing of DDX60L. Luciferase activity was normalized to the respective untreated controls. Shown are mean values with SDs (n = 2). (D) DDX60L knockdown does not influence translation of the HCV IRES. Huh-7-Lunet cells were transfected with siRNA targeting DDX60L and 48 h later electroporated with replication-deficient reporter replicons, relying on either the HCV or the unrelated poliovirus IRES for translation of FLuc. Capped in vitro transcripts encoding Renilla luciferase (RLuc) were cotransfected to normalize for transfection efficiency. FLuc and RLuc activity was determined in cell lysates 2 h after transfection. Shown are mean values with SDs (n = 2). (E) Stability of HCV RNA is not altered by DDX60L knockdown. LucubineoJFH1 cells were transduced with lentiviruses encoding shRNAs against DDX60L at an MOI of 5. Twenty-four hours later, the cells were treated with 100 μM 2′CMC to inhibit the de novo synthesis of viral RNA. At the indicated time points, total RNA was isolated to determine the amount of HCV genomes by qRT-PCR. Values were normalized to the amount of total RNA. Shown are mean values with SDs (n = 2).