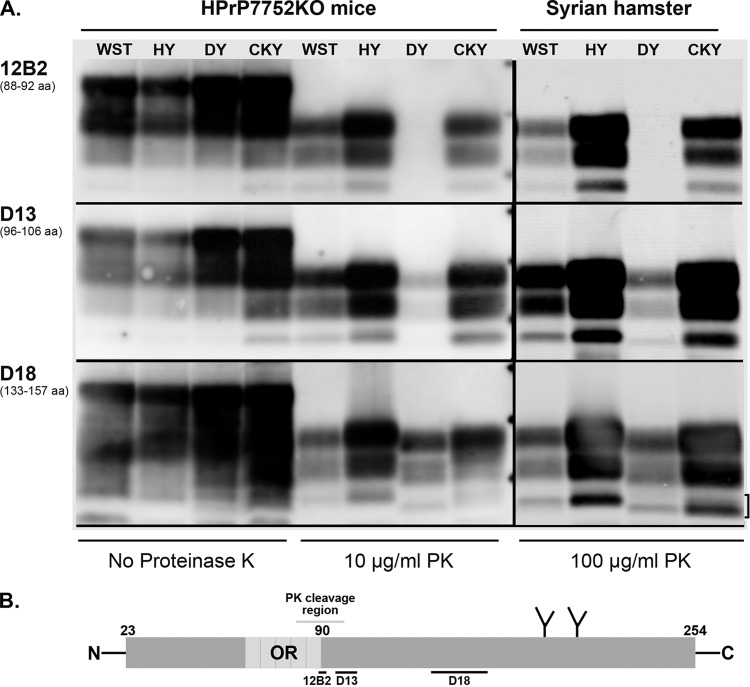
FIG 4.
Antigenic mapping of PrPSc from TME and CWD infection of transgenic mice and Syrian golden hamsters. (A) Prion-infected brain homogenates from HPrP7752KO mice and Syrian golden hamsters were digested with or without proteinase K (PK) and analyzed by SDS-PAGE and Western blotting with the anti-PrP antibodies 12B2, D13, and D18. The hamster amino acid (aa) sequence that is recognized (12B2) or used as a peptide antigen (D13 and D18) is indicated below each antibody. Brain tissues were from the second (CKY CWD) and third (WST CWD) serial passages in SGH. The three short horizontal bars between the PK-treated samples from HPrP7752KO mice and Syrian golden hamsters correspond to molecular masses of 20, 30, and 40 kDa. The bracket indicates the doublet PrPSc polypeptide found in CKY CWD. (B) Schematic of the linear amino acid map of the mature prion protein, including the octapeptide repeat (OR) region, two N-linked glycosylation sites, and carbohydrate structures (the Y-like branches). Limited PK digestion of PrPSc results in the degradation of the N-terminal region. This results in a ragged N terminus with new N termini around amino acid 90. The locations of the peptides used to generate the anti-PrP antibodies 12B2, D13, and D18 are indicated with horizontal bars below the prion protein map. Enzymatic deglycosylation of PK-treated PrPSc removes the two N-linked carbohydrate structures and results in an ∼25% reduction in molecular weight and a single major PrPSc polypeptide (see Fig. 5B).