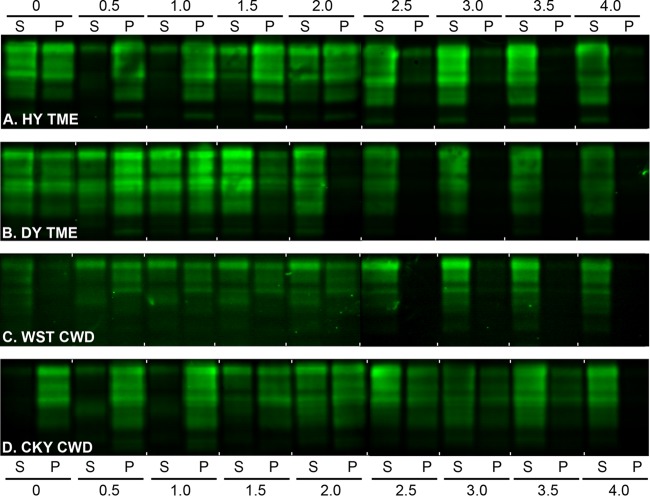
FIG 6.
Western blot of prion protein from the TME and CWD strains in Syrian golden hamsters after a conformation solubility and stability assay (CSSA). SGH brain homogenates from HY TME (A), DY TME (B), WST CWD (C), and CKY CWD (D) were extracted in Sarkosyl, incubated with GdnHCl from 0 to 4.0 M, and subjected to ultracentrifugation as described for the CSSA. For each GdnHCl treatment, the supernatant (S) and pellet (P) fraction after ultracentrifugation were analyzed in adjacent lanes by SDS-PAGE and Western blotting with anti-PrP D18 antibody. Brain tissue was from the second (CKY CWD) and third (WST CWD) serial passages in SGH. For each panel, two separate Western blots prepared for strain analysis, and for illustrative purposes, they were merged between the 2.0 and 2.5 M GdnHCl values.