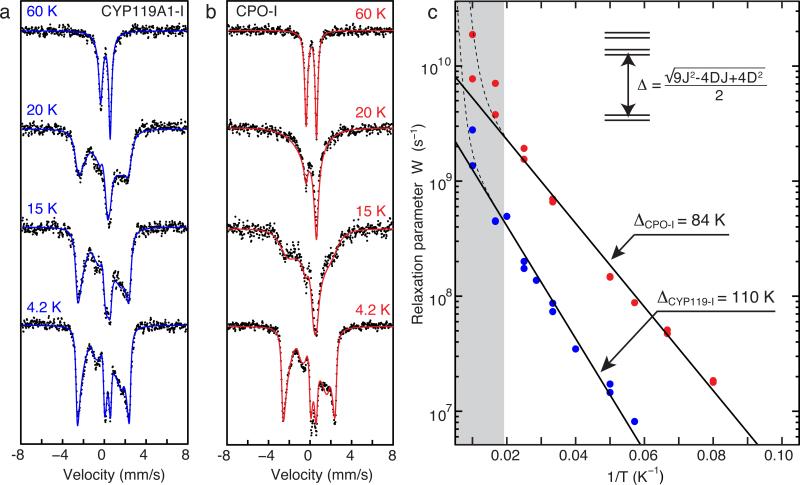
Figure 3.
Variable temperature Mössbauer data for (a) CYP119A1-I and (b) CPO-I. With increasing temperature, the Mössbauer spectrum of CPO-I collapses towards a quadrupole doublet more quickly than that of P450-I, indicating that P450-I has a slower electronic relaxation rate than CPO-I. Fits of the data shown in (c) reveal that a larger exchange coupling is responsible for the slower relaxation. The contributions from ferric enzyme were subtracted from each spectrum in (a). The splitting between the lowest Kramers doublets (Δ) was obtained by fitting the linear region in (c) to Eq. 1. The grey bar indicates the temperature range in which Raman relaxation modes begin to dominate the Orbach mechanism. A complete listing of Mössbauer paramaters and relaxation rates can be found in Supplementary Tables S1 and S2.