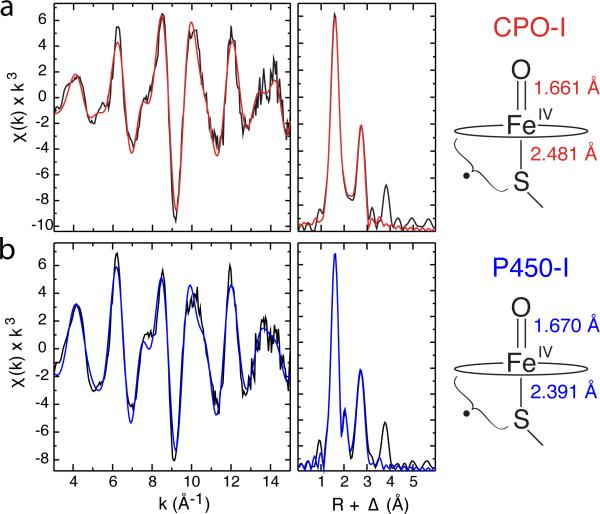
Figure 4.
Representative Fe K-edge EXAFS data and Fourier transforms for CPO-I (a) and CYP119A1-I (b). Raw data are shown in black and best fits are shown in red and blue, respectively. The distances shown at the right were obtained by averaging independent measurements for CPO-I and P450-I (CYP119A1-I, CYP119A2-I, and CYP158A2*-I). These distances reveal that the Fe-S bond in P450-I is significantly shorter than in CPO-I, suggesting more electron donation from the proximal thiolate ligand to the ferryl moiety in P450. This may in part explain P450's greater propensity for C-H bond activation.