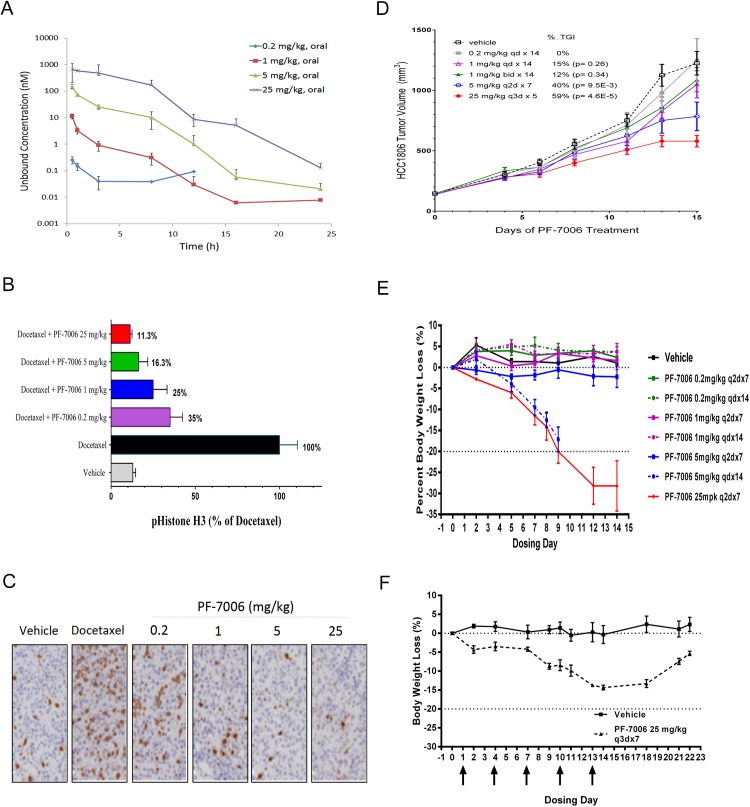
Fig 4. In vivo pharmacology of PF-7006 in the HCC1806 triple-negative breast cancer model.
(A) Plasma concentration of PF-7006 following oral dosing of SCID mice (n = 3) to PF-7006 (0.2, 1, 5, 25 mg/kg). Error bars are standard deviations. (B) Modulation of mechanistic marker (pHH3-Ser10) in response to PF-7006 treatment as established by ELISA assay (n = 6). Error bars are standard deviations. (C) Modulation of pHH3-Ser10 in response to PF-7006 treatment based on tumor IHC (n = 3). (D) Tumor growth inhibition from PF-7006 treatment of HCC1806 tumor-bearing mice as a function of dosing and scheduling (n = 12). The following schedules were tested: 0.2 mg/k QD x 14 days (0% TGI), 1 mg/kg QD x 14 days (15% TGI, p = 0.26), 1 mg/kg BID x 14 (12% TGI, p = 0.34), 5 mg/kg Q2D x 7 (40% TGI, p = 9.5x10-3), and 25 mg/kg Q3D x 5 (59% TGI, p = 4.6x10-5). Error bars are standard error of the mean. (E) Effect of different doses and schedules of PF-7006 administration on body weights (n = 3). The following schedules were evaluated: 0.2 mg/kg Q2Dx7, 0.2 mg/kg Q2Dx14, 1 mg/kg Q2Dx7, 1 mg/kg QD x14, 5 mg/kg Q2Dx7, 5 mg/kg QD x 14 (significant body weight loss), and 25 mg/kg Q2Dx7 (significant body weight loss). Error bars are standard error of the mean. F. Effect on body weight based on intermittent PF-7006 dosing (25 mg/kg Q3Dx7, n = 3). Upon cessation of dosing, treated animals regained body weight. Error bars are standard error of the mean.