Figure 1.
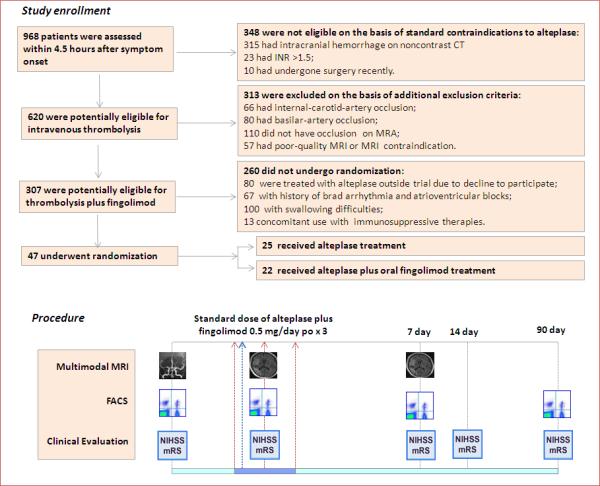
Study flow chart. A total of 968 patients with stroke-like symptoms underwent screening within 4.5 hours after symptom onset. The 620 patients potentially eligible for intravenous thrombolysis were further screened for participation in the study. 47 patients were randomly assigned in a 1:1 ratio to the standard dose of alteplase (0.9 mg per kg, the first 10% administered as an initial bolus and the remainder over a 1-hour period, with a maximum dose of 90 mg); or to the standard dose of alteplase immediately following the 1st dose of oral fingolimod (0.5 mg of FTY720 (Gilenya, Novartis). Fingolimod was administered once daily, for three consecutive days. Counts of circulating lymphocyte subsets were monitored by flow cytometry. Clinical assessments (NIHSS, mRS) were conducted at the indicated time points. Alterations of MRA status, lesion volume and hemorrhage volume were measured on MRI at the indicated time points.
