Figure. 2.
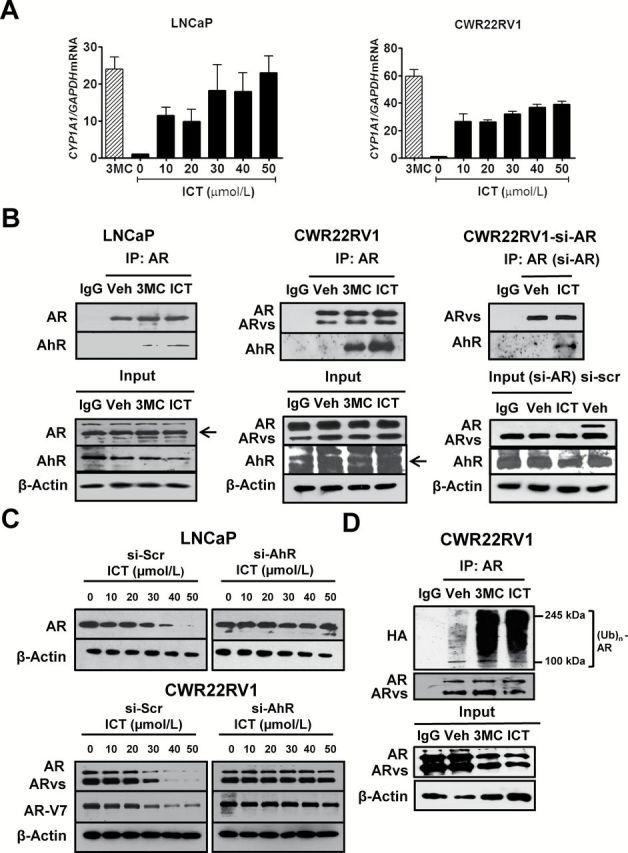
ICT promotes AR and AR splice variant protein degradation through AhR-mediated ubiquitin proteasome pathway. (A) LNCaP and CWR22Rv1 cells were treated with DMSO (Veh), 3MC (5 μmol/l) and ICT for 24h. CYP1A1 mRNA levels were analyzed using quantitative RT–PCR and normalized against 18S rRNA. Data shown are mean ± SEM of three independent experiments. (B) Co-IP of AhR with AR or ARvs. CWR22Rv1 cells were transiently transfected with siRNA targeted to AR exon 7 (si-AR) or si-Scr for 72h followed by drug treatments. Untransfected LNCaP and CWR22Rv1 and transfected CWR22Rv1 cells were treated with either DMSO (Veh), ICT (30 μmol/l) and/or 3MC (5 μmol/l) for 2h. AR- or ARvs-bound proteins were immunoprecipitated from cleared lysates using AR (co-targeting AR full-length and splice variants) or IgG antibody. The presence of AR, AR-V7 and AhR proteins (arrows) were identified using specific antibodies. (C) LNCaP and CWR22Rv1 cells were transiently transfected with siRNA against AhR (si-AhR) or si-Scr for 72h followed by 24h treatment with ICT. Whole cell lysates were then analysed by western blot for AR and AR-V7. β-actin was used as a loading control. (D) Ubiquitination of AR protein. CWR22Rv1 cells were transiently transfected with pCDNA3-HA-Ub for 48h, followed by 24h treatment with either DMSO (Veh), 3MC (5 μmol/l) or ICT (30 μmol/l). Co-IP and immunoblotting were performed, as described for (B), using antibodies against AR and HA tag.
