Abstract
Protein disulfide bond formation in Escherichia coli requires the periplasmic protein DsbA. We describe here mutations in the gene for a second protein, DsbB, which is also necessary for disulfide bond formation. Evidence suggests that DsbB may act by reoxidizing DsbA, thereby regenerating its ability to donate its disulfide bond to target proteins. We propose that DsbB, an integral membrane protein, may be involved in transducing redox potential across the cytoplasmic membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
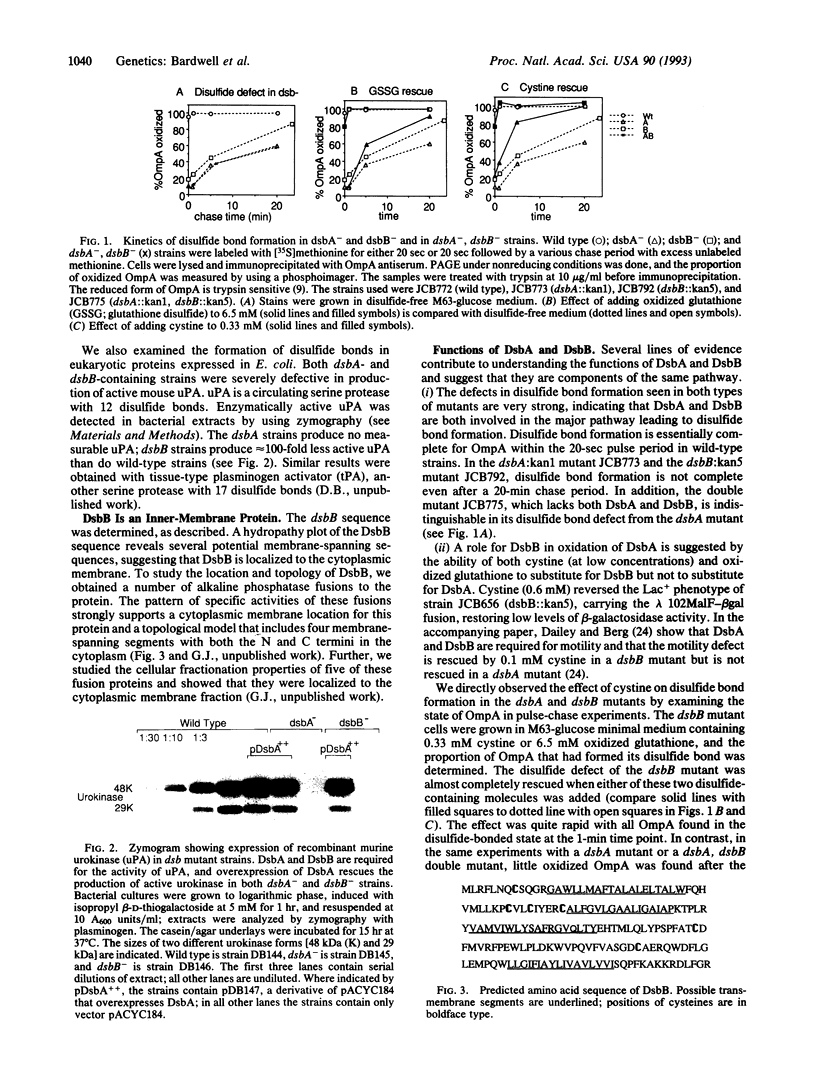
- Bardwell J. C., McGovern K., Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991 Nov 1;67(3):581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- Belin D., Vassalli J. D., Combépine C., Godeau F., Nagamine Y., Reich E., Kocher H. P., Duvoisin R. M. Cloning, nucleotide sequencing and expression of cDNAs encoding mouse urokinase-type plasminogen activator. Eur J Biochem. 1985 Apr 15;148(2):225–232. doi: 10.1111/j.1432-1033.1985.tb08829.x. [DOI] [PubMed] [Google Scholar]
- Dailey F. E., Berg H. C. Mutants in disulfide bond formation that disrupt flagellar assembly in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1043–1047. doi: 10.1073/pnas.90.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig A. J., Williams D. H. Is the hydrophobic effect stabilizing or destabilizing in proteins? The contribution of disulphide bonds to protein stability. J Mol Biol. 1991 Jan 20;217(2):389–398. doi: 10.1016/0022-2836(91)90551-g. [DOI] [PubMed] [Google Scholar]
- Duvoisin R. M., Belin D., Krisch H. M. A plasmid expression vector that permits stabilization of both mRNAs and proteins encoded by the cloned genes. Gene. 1986;45(2):193–201. doi: 10.1016/0378-1119(86)90254-4. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Fischer G., Schmid F. X. The mechanism of protein folding. Implications of in vitro refolding models for de novo protein folding and translocation in the cell. Biochemistry. 1990 Mar 6;29(9):2205–2212. doi: 10.1021/bi00461a001. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Protein folding: local structures, domains, subunits, and assemblies. Biochemistry. 1991 Apr 2;30(13):3147–3161. doi: 10.1021/bi00227a001. [DOI] [PubMed] [Google Scholar]
- Janolino V. G., Swaisgood H. E. Isolation and characterization of sulfhydryl oxidase from bovine milk. J Biol Chem. 1975 Apr 10;250(7):2532–2538. [PubMed] [Google Scholar]
- Kamitani S., Akiyama Y., Ito K. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J. 1992 Jan;11(1):57–62. doi: 10.1002/j.1460-2075.1992.tb05027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Bender J., Gottesman S. Uses of transposons with emphasis on Tn10. Methods Enzymol. 1991;204:139–180. doi: 10.1016/0076-6879(91)04009-d. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Peek J. A., Taylor R. K. Characterization of a periplasmic thiol:disulfide interchange protein required for the functional maturation of secreted virulence factors of Vibrio cholerae. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6210–6214. doi: 10.1073/pnas.89.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner E., Padan E., Schuldiner S. Cloning, sequencing, and expression of the nhaB gene, encoding a Na+/H+ antiporter in Escherichia coli. J Biol Chem. 1992 Jun 5;267(16):11064–11068. [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Knobler R. L., Buchmeier M. J. Western and dot immunoblotting analysis of viral antigens and antibodies: application to murine hepatitis virus. J Immunol Methods. 1984 Oct 12;73(1):177–188. doi: 10.1016/0022-1759(84)90043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y., Kuroki R., Omura F., Seko C., Kikuchi M. Evidence for intramolecular disulfide bond shuffling in the folding of mutant human lysozyme. J Biol Chem. 1991 Apr 5;266(10):6456–6461. [PubMed] [Google Scholar]
- Tomb J. F. A periplasmic protein disulfide oxidoreductase is required for transformation of Haemophilus influenzae Rd. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10252–10256. doi: 10.1073/pnas.89.21.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Webb H., Hirst T. R. A homologue of the Escherichia coli DsbA protein involved in disulphide bond formation is required for enterotoxin biogenesis in Vibrio cholerae. Mol Microbiol. 1992 Jul;6(14):1949–1958. doi: 10.1111/j.1365-2958.1992.tb01368.x. [DOI] [PubMed] [Google Scholar]