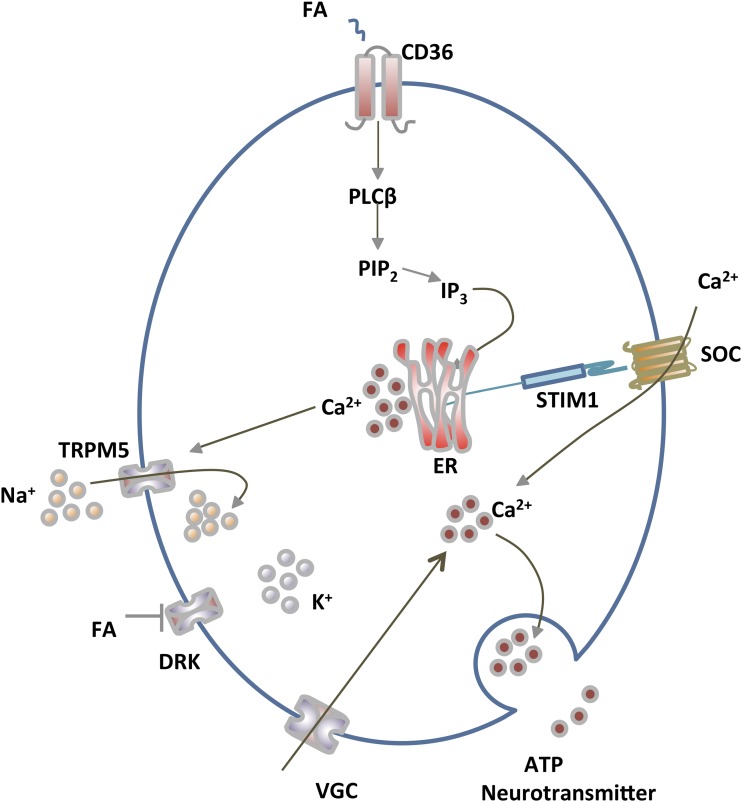
FIGURE 2.
CD36-mediated FA signaling for fat taste perception in TBCs. Long-chain FAs (mainly unsaturated) that bind to CD36 on TBCs activate phospholipase C to generate IP3, which interacts with its receptors on the ER to release Ca2+. ER Ca2+ depletion is sensed by STIM1, which activates membrane SOC influx. Gilbertson and Khan (28) provided evidence that the FAs also induce membrane depolarization by activating inward sodium flux via TRPM5 (and possibly inhibition of DRK-mediated potassium efflux), resulting in VGC influx. The sustained rise in cytosolic Ca2+ culminates in neurotransmitter release and relay of fat taste perception to brain centers (28). The 2 FA receptors identified on TBCs in rodents and humans are CD36 and GPR120. CD36 is the physiologically functional receptor (15). GPR120 might be important for release of GLP-1 and for amplifying signaling at high FA amounts. DRK, delayed rectifying K+ channel; ER, endoplasmic reticulum; GLP, glucagon-like peptide; GPR, G protein-coupled receptor; IP3, inositol-1,4,5-triphosphate; PIP2, phosphatidylinositol-4,5-bisphosphate; PLC, phospholipase C; SOC, store-operated Ca2+; STIM1, stromal interaction molecule 1; TBC, taste bud cell; TRMP5: transient receptor potential M5; VGC, voltage-gated Ca2+. Adapted from reference 18 with permission.