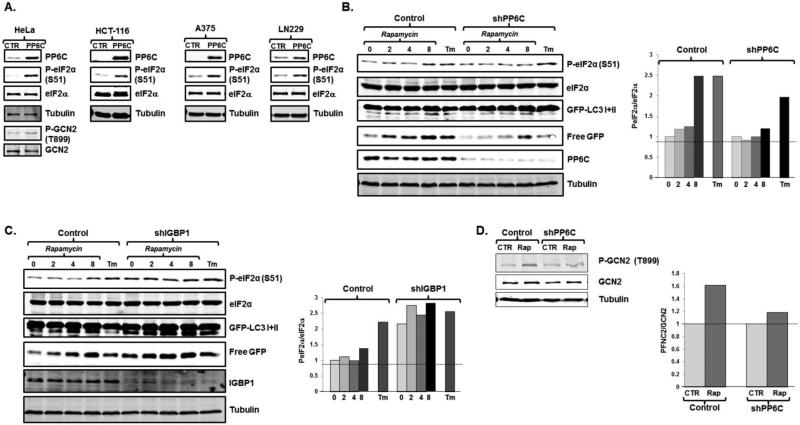
Fig 4.
Rapamycin leads to eIF2α phosphorylation and autophagy through activation of PP6C. A. HCT-116 and HeLa cells stably expressing a control retroviral vector or a vector expressing PP6C were assessed for GCN2 and/or eIF2α phosphorylation. Representative blots are displayed (N=2 biological replicates). B. U2OS cells stably expressing either a control, or shPP6C lentivirus were treated with 300nM rapamycin for the times indicated (hours) or tunicamycin for 12 hours. Protein lysates were then immunoblotted for phosphorylated eIF2α and other noted proteins. A representative blot is displayed (left) and quantitation reflects average of two biological replicates (right). C. HeLa cells stably expressing either a control, or shIGBP1 lentivirus were treated with 300nM rapamycin for the times indicated (hours) or tunicamycin for 12 hours. Protein lysates were then immunoblotted, with a representative blot (left) and quantitation reflective of the average of two biological replicates (right). D. Scramble (con) and PP6C depleted cells were treated with rapamycin for 6 hours and phosphorylated and total GCN2 levels were assessed. A representative blot is displayed (left) and quantitation reflects average of two biological replicates (right).