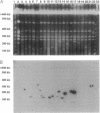
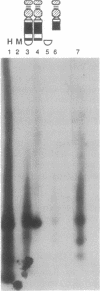
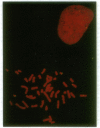
Abstract
Construction of chromosome-specific yeast artificial chromosome (YAC) libraries from sorted chromosomes was undertaken (i) to eliminate drawbacks associated with first-generation total genomic YAC libraries, such as the high frequency of chimeric YACs, and (ii) to provide an alternative method for generating chromosome-specific YAC libraries in addition to isolating such collections from a total genomic library. Chromosome-specific YAC libraries highly enriched for human chromosomes 16 and 21 were constructed. By maximizing the percentage of fragments with two ligatable ends and performing yeast transformations with less than saturating amounts of DNA in the presence of carrier DNA, YAC libraries with a low percentage of chimeric clones were obtained. The smaller number of YAC clones in these chromosome-specific libraries reduces the effort involved in PCR-based screening and allows hybridization methods to be a manageable screening approach.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abidi F. E., Wada M., Little R. D., Schlessinger D. Yeast artificial chromosomes containing human Xq24-Xq28 DNA: library construction and representation of probe sequences. Genomics. 1990 Jul;7(3):363–376. doi: 10.1016/0888-7543(90)90170-y. [DOI] [PubMed] [Google Scholar]
- Bartholdi M., Meyne J., Albright K., Luedemann M., Campbell E., Chritton D., Deaven L. L., Cram L. S. Chromosome sorting by flow cytometry. Methods Enzymol. 1987;151:252–267. doi: 10.1016/s0076-6879(87)51022-9. [DOI] [PubMed] [Google Scholar]
- Bronson S. K., Pei J., Taillon-Miller P., Chorney M. J., Geraghty D. E., Chaplin D. D. Isolation and characterization of yeast artificial chromosome clones linking the HLA-B and HLA-C loci. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1676–1680. doi: 10.1073/pnas.88.5.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers P. M., Percival K. J. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987 Jun;163(2):391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Rigault P., Guillou S., Ougen P., Billaut A., Guasconi G., Gervy P., LeGall I., Soularue P., Grinas L. Continuum of overlapping clones spanning the entire human chromosome 21q. Nature. 1992 Oct 1;359(6394):380–387. doi: 10.1038/359380a0. [DOI] [PubMed] [Google Scholar]
- Connelly C., McCormick M. K., Shero J., Hieter P. Polyamines eliminate an extreme size bias against transformation of large yeast artificial chromosome DNA. Genomics. 1991 May;10(1):10–16. doi: 10.1016/0888-7543(91)90477-v. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Van Dilla M. A., Bartholdi M. F., Carrano A. V., Cram L. S., Fuscoe J. C., Gray J. W., Hildebrand C. E., Moyzis R. K., Perlman J. Construction of human chromosome-specific DNA libraries from flow-sorted chromosomes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):159–167. doi: 10.1101/sqb.1986.051.01.019. [DOI] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. D., Riethman H. C., Dutchik J. E., Olson M. V. Detection and characterization of chimeric yeast artificial-chromosome clones. Genomics. 1991 Nov;11(3):658–669. doi: 10.1016/0888-7543(91)90073-n. [DOI] [PubMed] [Google Scholar]
- Hieter P., Mann C., Snyder M., Davis R. W. Mitotic stability of yeast chromosomes: a colony color assay that measures nondisjunction and chromosome loss. Cell. 1985 Feb;40(2):381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Lawrence J. B., Ruddle F. H. A sequential staining technique for the chromosomal analysis of the interspecific mouse/hamster and mouse/human somatic cell hybrids. Exp Cell Res. 1977 Mar 1;105(1):109–117. doi: 10.1016/0014-4827(77)90156-2. [DOI] [PubMed] [Google Scholar]
- Little R. D., Porta G., Carle G. F., Schlessinger D., D'Urso M. Yeast artificial chromosomes with 200- to 800-kilobase inserts of human DNA containing HLA, V kappa, 5S, and Xq24-Xq28 sequences. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1598–1602. doi: 10.1073/pnas.86.5.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M. K., Shero J. H., Cheung M. C., Kan Y. W., Hieter P. A., Antonarakis S. E. Construction of human chromosome 21-specific yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9991–9995. doi: 10.1073/pnas.86.24.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Moyzis R. K., Meyne J., Burke D. T., Olson M. V. Cloning human telomeric DNA fragments into Saccharomyces cerevisiae using a yeast-artificial-chromosome vector. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6240–6244. doi: 10.1073/pnas.86.16.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shero J. H., McCormick M. K., Antonarakis S. E., Hieter P. Yeast artificial chromosome vectors for efficient clone manipulation and mapping. Genomics. 1991 Jun;10(2):505–508. doi: 10.1016/0888-7543(91)90343-d. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Torney D. C., Hildebrand C. E., Longmire J. L., Deaven L. L., Jett J. H., Doggett N. A., Moyzis R. K. Physical mapping of human chromosomes by repetitive sequence fingerprinting. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6218–6222. doi: 10.1073/pnas.87.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dilla M. A., Deaven L. L. Construction of gene libraries for each human chromosome. Cytometry. 1990;11(1):208–218. doi: 10.1002/cyto.990110124. [DOI] [PubMed] [Google Scholar]
- Wada M., Little R. D., Abidi F., Porta G., Labella T., Cooper T., Della Valle G., D'Urso M., Schlessinger D. Human Xq24-Xq28: approaches to mapping with yeast artificial chromosomes. Am J Hum Genet. 1990 Jan;46(1):95–106. [PMC free article] [PubMed] [Google Scholar]