Abstract
Bone morphogenic protein and activin membrane-bound inhibitor (BAMBI) is regarded as an essential regulator of cell proliferation and differentiation that represses transforming growth factor-β and enhances Wnt/β-catenin signaling in various cell types. However, its role in skeletal muscle remains largely unknown. In the current study, we found that the expression level of BAMBI peaked in the early differentiation phase of the C2C12 rodent myoblast cell line. Knockdown of BAMBI via siRNA inhibited C2C12 differentiation, indicated by repressed MyoD, MyoG, and MyHC expression as well as reductions in the differentiation and fusion indices. BAMBI knockdown reduced the activity of Wnt/β-catenin signaling, as characterized by the decreased nuclear translocation of β-catenin and the lowered transcription of Axin2, which is a well-documented target gene of the Wnt/β-catenin signaling pathway. Furthermore, treatment with LiCl, an activator of Wnt/β-catenin signaling, rescued the reduction in C2C12 differentiation caused by BAMBI siRNA. Taken together, our data suggest that BAMBI is required for normal C2C12 differentiation, and that its role in myogenesis is mediated by the Wnt/β-catenin pathway.
Keywords: BAMBI, Wnt/β-catenin, myogenic differentiation, LiCl, C2C12
1. Introduction
Bone morphogenic protein and activin membrane-bound inhibitor (BAMBI) is a transmembrane protein composed of 261 amino acids in mice, which shares high similarity with the transforming growth factor-β (TGF-β) family type I receptor in their extracellular domains, while lacking an intracellular kinase domain [1,2]. BAMBI has been reported to promote the invasion, migration, and proliferation of cancer cells [3,4], and to suppress adipogenesis [5,6]. Previous studies have shown that BAMBI significantly inhibits the expression of carcinoma-associated fibroblast markers in human bone marrow mesenchymal stem cells without affecting their stem cell and tumor-tropic properties [7].
BAMBI is involved in several signaling pathways. BAMBI can interfere with the association between the TGF-β type I and type II receptors [1] and inhibit the interaction between TGF-β receptor I and Smad3 as a decoy receptor in mouse embryonic carcinoma P19 cells and human embryonic kidney HEK293T cells [2], respectively. BAMBI also promotes the activity of Wnt/β-catenin signaling in porcine preadipocytes [5], HEK293T cells [8], and Simpson–Golabi–Behmel syndrome preadipocytes [6]. Moreover, BAMBI has been proposed to mediate the inductive effect of fibroblast growth factor on the expression of the sonic hedgehog gene during limb regeneration [9].
Wnt/β-catenin signaling plays an essential role during embryonic muscle development and adult skeletal muscle homeostasis. Wnt signaling activity promotes the expansion and differentiation of myogenic progenitors during lineage specification and muscle regeneration [10]. R-spondins, which have been characterized as Wnt/β-catenin signaling activators, promote myogenesis and induce hypertrophic myotube formation in C2C12 cells [11,12]. Glycogen synthase kinase-3β is a negative regulator of Wnt/β-catenin signaling, and inhibiting this kinase enhances the myogenic differentiation of C2C12 cells [13,14].
Given that BAMBI is involved in regulating the proliferation and differentiation of multiple cell types, and that Wnt/β-catenin signaling is essential for myogenic differentiation, we proposed that BAMBI might influence C2C12 differentiation, and that Wnt/β-catenin signaling might also be involved. In our studies, we blocked BAMBI expression using siRNA and found that BAMBI plays essential roles in C2C12 myogenic differentiation and Wnt/β-catenin signaling transduction.
2. Results
2.1. Expression Patterns of BAMBI
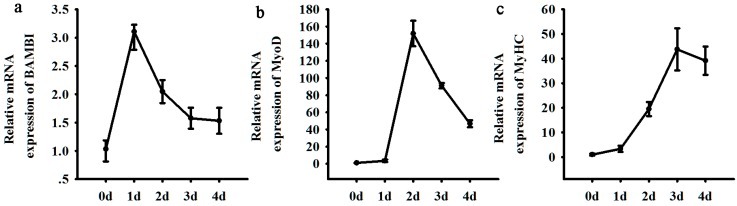
Our data showed that BAMBI mRNA expression increased three-fold on day 1 of myogenesis compared with its expression in undifferentiated cells on day 0, and then gradually declined in the following days (Figure 1a). The expression of MyoD, a well-known myogenic marker, peaked on day 2 post differentiation (Figure 1b). The peak of BAMBI expression was observed prior to that of MyoD expression, indicating that BAMBI might play a role during the early phase of C2C12 differentiation. The differentiation status of C2C12 cells was monitored by measuring the expression levels of MyoD and MyHC, which both consistently increased over time (Figure 1b,c).
Figure 1.
The temporal mRNA expression profiles of BAMBI (a), MyoD (b) and MyHC (c) during C2C12 myogenic differentiation. The results were represented as mean ± SD.
2.2. Knockdown of BAMBI Inhibits C2C12 Myogenic Differentiation
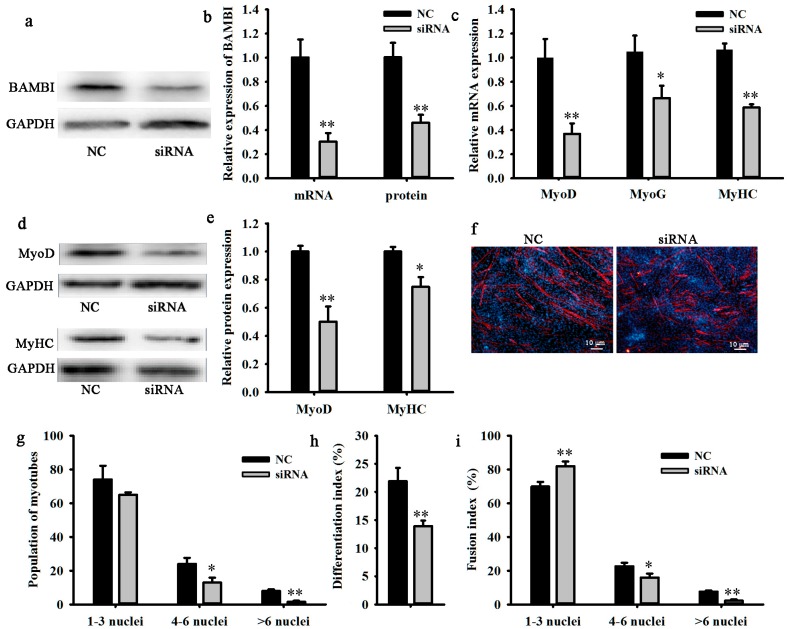
To explore the role of BAMBI during C2C12 myogenic differentiation, siRNA was used to interfere with the expression of BAMBI. Results demonstrated that BAMBI siRNA significantly inhibited both the mRNA and protein expression of BAMBI at 48 h post transfection and induction (Figure 2a,b). Meanwhile, the expression levels of MyoD at 48 h as well as those of MyoG and MyHC at 96 h were significantly suppressed (Figure 2c–e). The population of myotubes was obviously reduced, especially in the myotubes with 4–6 nuclei and >6 nuclei (Figure 2f,g). The differentiation and fusion indices were also decreased (Figure 2h,i). These results suggest that the interference of BAMBI expression inhibits the differentiation of C2C12 cells.
Figure 2.
Knockdown of BAMBI inhibited myogenic differentiation. All the cell samples were harvested after transfection and myogenic induction for 48 and 96 h. (a) The western blot images of BAMBI and GAPDH; (b) the efficiency of siRNA interference on the mRNA and protein expression of BAMBI; (c) the mRNA expression of MyoD at 48 h and that of MyoG and MyHC at 96 h; (d) the western blot images of MyoD at 48 h, MyHC at 96 h, and their corresponding GAPDH; (e) the protein expression of MyoD at 48 h and MyHC at 96 h; (f) immunofluorescence of MyHC in C2C12 myotubes at 96 h post differentiation, images captured at 100× magnification; (g) the populations of myotubes; (h) the differentiation index; and (i) the myotube fusion index. The results were represented as mean ± SD; n = 3; * p < 0.05; ** p < 0.01.
2.3. Knockdown of BAMBI Inhibits Wnt/β-Catenin Signaling during C2C12 Differentiation
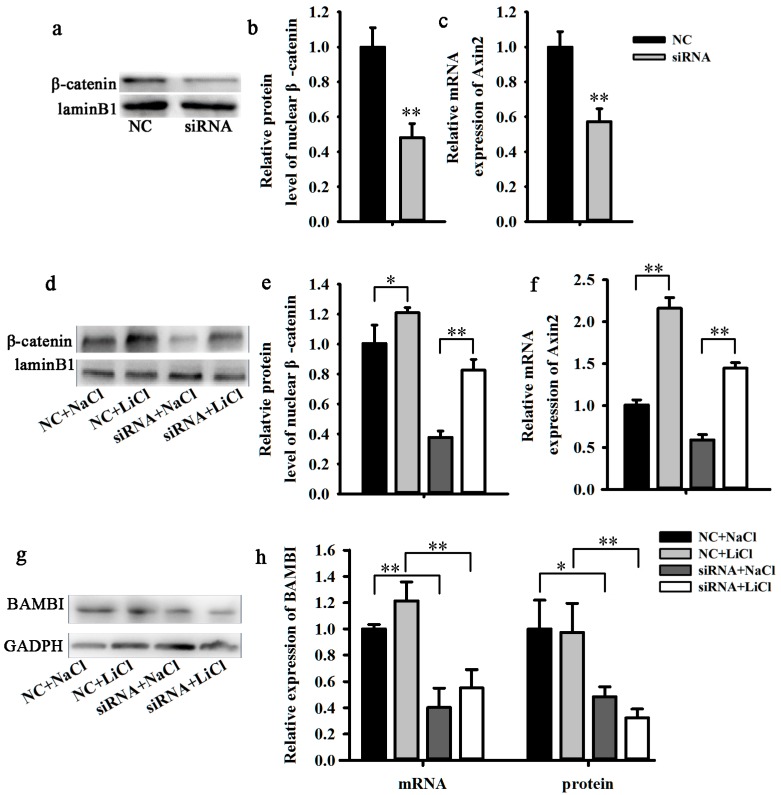
In order to investigate the relationship between BAMBI and Wnt/β-catenin signaling transcription activity in C2C12 cells, we measured Axin2 transcription and the nuclear translocation of β-catenin, which are two indicators for Wnt/β-catenin activity. At 48 h post induction, BAMBI siRNA decreased these two indicators significantly (Figure 3a–c). Subsequently, we activated Wnt/β-catenin signaling using 10 mM LiCl, and found that LiCl restored the influence of BAMBI on Wnt/β-catenin signaling (Figure 3d–f) but did not alter the expression of BAMBI (Figure 3g,h).
Figure 3.
Knockdown of BAMBI suppressed Wnt/β-catenin signaling. All the cell samples were harvested after transfection and myogenic induction for 48 h. (a) The western blot images of nuclear β-catenin and laminB1; (b) the nuclear β-catenin protein levels; (c) the mRNA expression of Axin2; (d) the western blot images of nuclear β-catenin and laminB1; (e) the nuclear β-catenin protein levels; (f) the mRNA expression of Axin2; (g) the western blot images of BAMBI and GAPDH; and (h) the mRNA and protein expression of BAMBI. The results were represented as mean ± SD; n = 3; * p < 0.05; ** p < 0.01.
2.4. LiCl Rescued the Influence of BAMBI Interference on C2C12 Differentiation
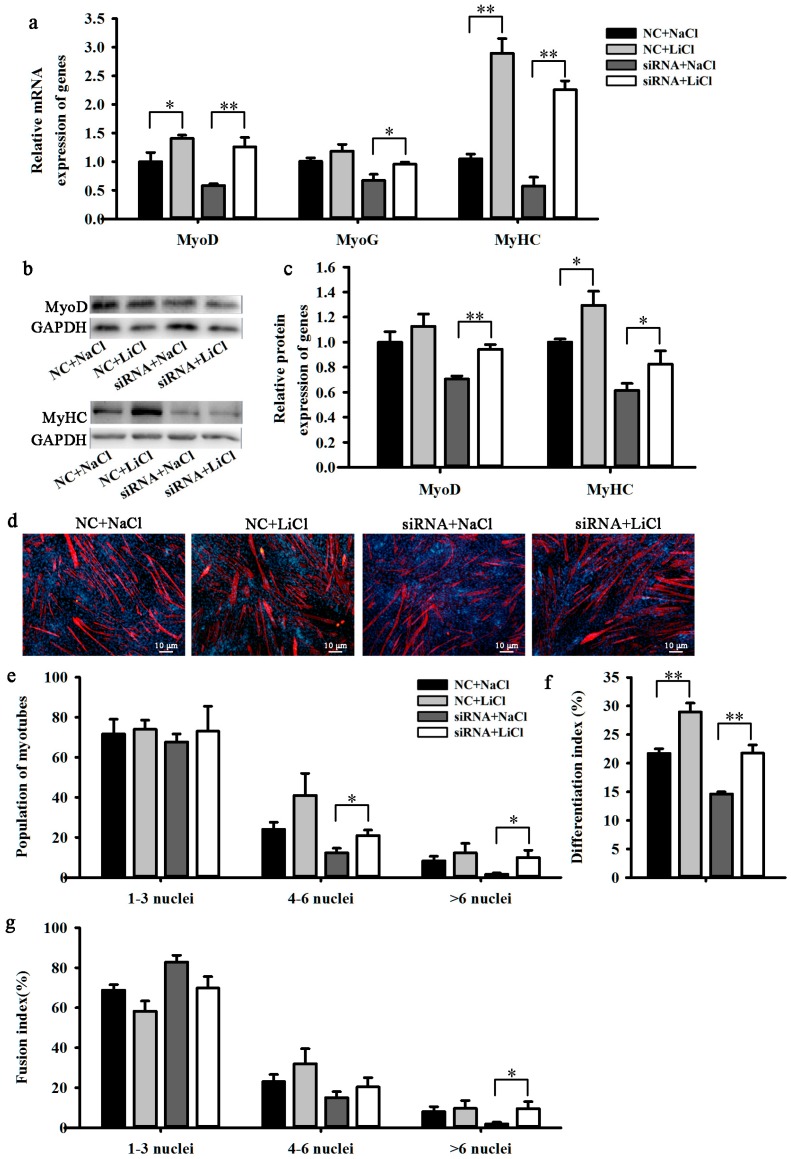
The expression of BAMBI was reduced significantly by treatment with siRNA in media containing 10 mM LiCl or NaCl (Figure 3g,h). In the presence of NC, LiCl promoted the expression of MyoD, MyoG, and MyHC compared with their expression in cells treated with NaCl (Figure 4a–c). The population of myotubes and the differentiation and fusion indices were increased significantly (Figure 4d–g). In the presence of siRNA, LiCl rescued the negative influence of the knockdown of BAMBI on the above indicators (Figure 4). These results suggest that BAMBI promotes C2C12 myogenic differentiation by enhancing Wnt/β-catenin signaling.
Figure 4.
LiCl rescued the inhibitory effect of BAMBI siRNA on C2C12 myogenic differentiation. All the cell samples were harvested after transfection and myogenic induction for 48 and 96 h. (a) The mRNA expression of MyoD at 48 h and that of MyoG and MyHC at 96 h; (b) the western blot images of MyoD, MyHC, and GAPDH; (c) the protein expression of MyoD at 48 h and MyHC at 96 h; (d) immunofluorescence images of MyHC in C2C12 myotubes at 96 h post differentiation, images captured at 100× magnification; (e) the populations of myotubes; (f) the differentiation index and (g) the myotube fusion index. The results were represented as mean ± SD; n = 3; * p < 0.05; ** p < 0.01.
3. Discussion
Skeletal muscle development is a highly orchestrated biological process, involving myogenic progenitor cell maintenance, lineage specification, proliferation, and terminal differentiation. Wnt signaling participates in the regulation of embryonic muscle development and adult muscle regeneration [15,16]. During the onset of C2C12 myogenic differentiation, the expression of BAMBI was strongly induced prior to that of MyoD, and then it gradually declined. Knockdown of BAMBI using siRNA impaired the expression of MyoD, MyoG, and MyHC and the formation of myotubes, indicating that BAMBI plays an essential role during C2C12 myogenic differentiation.
Canonical Wnt proteins mainly promote myogenic lineage specification, mainly via inducing the activity of myogenic regulatory factors by way of the β-catenin/T-cell factors (TCFs) transcriptional complex [17]. Wnt3a contributes to the post-translational regulation and activation of MyoD and MyoG during myogenesis in P19 embryonal carcinoma stem cells [18]. MyoD is a direct target of Wnt/β-catenin in myogenesis [19]. Nuclear β-catenin interacts with MyoD and enhances its binding ability to the E-box element and its transcriptional activity [20].
It is well known that BAMBI exerts its functions through several signaling pathways, such as blocking TGF-β/Smad [7] and activating Wnt/β-catenin or extracellular signal-related kinase 1/2 signaling [5,21]. BAMBI facilitates the interaction between Frizzled5 and Dishevelled2, thus promoting canonical Wnt activity [8]. We also found that the experimental reduction of BAMBI expression significantly inhibited the nuclear translocation of β-catenin and the transcription of Axin2, two indicators of Wnt/β-catenin activity [22,23,24]. Similar results were observed in gastric cancer cells and preadipocytes [3,5,6]. The inhibitory effects of BAMBI knockdown on myogenic differentiation and Wnt/β-catenin signaling were reversed by supplementation with LiCl, an activator of Wnt/β-catenin activity [25,26]. These results show that Wnt/β-catenin signaling is a mediator of BAMBI’s role during C2C12 myogenic differentiation.
Notably, we did not observe any change in BAMBI expression following the treatment of C2C12 cells with LiCl, in accordance with previous results in Simpson–Golabi–Behmel syndrome preadipocytes [6], although BAMBI was previously identified as a downstream target of Wnt/β-catenin signaling in several other cell types [5,27]. These discrepancies may be due to differences among the cell models studied.
To further elucidate the mechanisms of BAMBI’s functions in regulating myogenic differentiation, direct knockdown of TCFs or specific inhibitors against the physical interaction between β-catenin and TCFs may be among the ideal methods to use in future research. Wnt signaling represents a gigantic regulatory network that consists of more than 40 components, and the relationship between myogenesis and Wnt signaling is far from being fully elucidated [15,16]. The ligands for Wnt signaling, such as Wnt3a, may act in a dose-dependent manner on C2C12 differentiation [24,28,29]. Nonetheless, the β-catenin/TCFs complex has unambiguous functions in C2C12 differentiation.
4. Experimental Section
4.1. Cell Culture
C2C12 cells [30] (ATCC, Manassas, VA, USA) were grown in DMEM (Invitrogen, Carlsbad, CA, USA) medium supplemented with 10% (v/v) fetal bovine serum (Hyclone, Logan, UT, USA), 2 mM L-glutamine, and 1% (v/v) penicillin/streptomycin solution at 37 °C and 5% CO2. When the cell density reached 90%, the growth medium was replaced with differentiation medium, which was DMEM medium containing 2% (v/v) horse serum (Invitrogen), 2 mM L-glutamine, and 1% (v/v) penicillin/streptomycin solution. All the used cells were at similar passages (P5–7). Media were changed every two days. LiCl (Solarbio, Beijing, China) was diluted to 1 M using ultrapure water.
4.2. Transfection of BAMBI siRNA and the siRNA Negative Control
BAMBI siRNA and a scrambled negative control (NC) were designed and purchased from Invitrogen (Table 1). When the cell density reached 70%, the C2C12 myoblasts were subjected to starvation in Opti-MEM® I reduced serum medium (Invitrogen) for 4 h prior to transfection. Lipofectamine® 2000 transfection reagent (Invitrogen) and siRNA or NC were gently mixed according to the manufacturer’s protocol, and then added to the culture supernatant. After 6 h of incubation, Opti-MEM I medium was replaced with fresh DM.
Table 1.
siRNA targeting the coding region of mouse BAMBI.
| siRNA | Sequences (5′–3′) | |
|---|---|---|
| BAMBI siRNA | Sense | GCAAGCAGAGCUCAGUAAUTT |
| Antisense | AUUACUGAGCUCUGCUUGCTT | |
| Negative control | Sense | GCAAGCAGAGCUCAGUAAUTT |
| Antisense | ACGUGACACGUUCGGAGAATT | |
4.3. Real-Time Quantitative PCR
Trizol® reagent (TaKaRa Bio, Otsu Japan) was applied to extract cellular total RNA. The RNA quality and concentration were estimated using agarose gel electrophoresis and spectrophotometry (Nanodrop, Wilmington, DE, USA), respectively. The total RNA was processed into single stranded cDNA using a reverse transcription kit (TaKaRa Bio). Real-time quantitative PCR was performed using a SYBR® green kit (TaKaRa Bio) on a Bio-Rad iQTM5 system (Bio-Rad, Hercules, CA, USA). GAPDH was used as a housekeeping gene for normalizing the expression of other genes. The 2−∆∆Ct algorithm was employed to estimate the relative expression level of each gene. The sequences of primers can be found in Table 2.
Table 2.
Specific primers for real-time PCR.
| Gene | Sequences (5′–3′) | Accession No. |
|---|---|---|
| MyoD | F: CATTCCAACCCACAGAACCT | NM_010866.1 |
| R: CAAGCCCTGAGAGTCGTCTT | ||
| MyoG | F: CAATGCACTGGAGTTCGGT | NM_031189.2 |
| R: CTGGGAAGGCAACAGACAT | ||
| MyHC | F: CGCAAGAATGTTCTCAGGCT | NM_030679.1 |
| R: GCCAGGTTGACATTGGATTG | ||
| Axin2 | F: CGCTCGGGTTTGTGTTAAGT | NM_015732.4 |
| R: GTCAACGCTCTGCCCTACAC | ||
| BAMBI | F: AAGCCTCAGGACAAGGAAA | NM_026505.2 |
| R: CAATGGGAACCGCTATCA | ||
| GAPDH | F: AACTTTGGCATTGTGGAAGG | NM_008084.3 |
| R: ACACATTGGGGGTAGGAACA |
4.4. Western Blot Detection
Total and nuclear proteins of C2C12 cells were extracted using kits from Vazyme, Nanjing, China. The protein concentration was determined using the bicinchoninic acid assay kit (Vazyme), and then 5× protein loading buffer was added to the lysates prior to full denaturation in boiled water for 10 min. A total of 20 µg of protein was electrophoresed on a 12% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Cell Signaling Technology, Danvers, MA, USA). The membrane was blocked in 5% (w/v) defatted milk at room temperature for 2 h, and then incubated at 4 °C overnight with primary antibodies, including MyoD (1:300 in Tris-buffered-saline containing Tween® 20 (TBST), sc-760, Santa Cruz Biotechnology, Dallas, TX, USA), BAMBI, MyHC (AF2387, MAB4470, 1:400 in TBST, R&D Systems, Minneapolis, MN, USA), β-catenin (1:400 in TBST, bs-1165R, Bioss, Beijing, China), laminB1 (ab16048, 1:1000 in TBST, Abcam, Cambridge, UK), and GAPDH (1:400 in TBST, BA2913, Boster, Wuhan, China). The next day, the membranes were washed with TBST and incubated with goat anti-mouse or anti-rabbit secondary antibodys (BA1050, BA1054, 1:1000 in TBST, Boster, Wuhan, China) at room temperature for 2 h. The membranes were washed three times with TBST and then exposed using a ChemiDoc XRS imaging system (Bio-Rad). The captured images were analyzed by Quantity One 4.6.3 software (Bio-Rad). The protein level of whole cell lysates was normalized against the expression of GAPDH, whereas the protein level of nuclear lysates was normalized against the expression of laminB1.
4.5. Immunocytochemical Analysis
Four days post myogenic differentiation, C2C12 cells were washed with cold PBS and fixed with 4% (w/v) paraformaldehyde for 15 min. 0.5% (v/v) Triton™ X-100 was used for permeabilization. The cells were then blocked in 0.5% (w/v) bovine serum albumin diluted in PBS. After blocking, the cells were incubated first with anti-MyHC antibody (1:250 in TBST, MAB4470, R&D Systems) at 37 °C for 2 h, and then with red fluorescence-labeled secondary antibody (a-11079, Invitrogen) at 37 °C for 1 h. The nuclei were stained with DAPI (1:1000 in PBS, 10236276001, Roche, Basel, Switzerland) for 10 min. Images were captured using a Nikon TE2000 microscope at 100× magnification (Nikon, Tokyo, Japan). The numbers of myotubes with 1–3, 4–6, and >6 nuclei were counted and averaged among three images per treatment at 100× magnification. The differentiation index was determined as the percentage of MyHC-positive nuclei among total nuclei, and the myotube fusion index was determined as the distribution of the nucleus number in total myotubes [12].
4.6. Statistical Analysis
Statistical analyses were performed in SPSS 19.0 statistical software (IBM, Armonk, NY, USA) using the Student t-test. Data are expressed as the mean ± SD. Statistical significance was indicated as follows: * p < 0.05; ** p < 0.01.
5. Conclusions
Our studies identify that the BAMBI is an essential and positive regulator of C2C12 differentiation, and Wnt/β-catenin signaling mediates its role during myogenic differentiation.
Acknowledgments
These studies were supported by “Major Projects for Genetically Modified Organisms Breeding” (Grant No. 2014ZX08009-047B) and “Specialized Research Fund for the Doctoral Program of Higher Education” (Grant No. 20120204110006).
Author Contributions
Conceived and designed the experiments: Xiao Li, Qiangling Zhang. Performed the experiments: Qiangling Zhang, Chengchuang Song. Analyzed the data and wrote the paper: Xiao Li, Qiangling Zhang. Our studies were also introduced by Xin-E Shi, Shiduo Sun, and Gongshe Yang.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Onichtchouk D., Chen Y.G., Dosch R., Gawantka V., Delius H., Massague J., Niehrs C. Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 2.Yan X., Lin Z., Chen F., Zhao X., Chen H., Ning Y., Chen Y.G. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-β signaling. J. Biol. Chem. 2009;284:30097–30104. doi: 10.1074/jbc.M109.049304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K., Song X., Ma H., Liu L., Wen X., Yu J., Wang L., Hu S. Knockdown of BAMBI inhibits β-catenin and transforming growth factor β to suppress metastasis of gastric cancer cells. Mol. Med. Rep. 2014;10:874–880. doi: 10.3892/mmr.2014.2305. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L., Park J., Jang K.Y., Park H.S., Wagle S., Yang K.H., Lee K., Park B., Kim J.R. The overexpression of BAMBI and its involvement in the growth and invasion of human osteosarcoma cells. Oncol. Rep. 2013;30:1315–1322. doi: 10.3892/or.2013.2569. [DOI] [PubMed] [Google Scholar]
- 5.Mai Y., Zhang Z., Yang H., Dong P., Chu G., Yang G., Sun S. BMP and activin membrane-bound inhibitor (BAMBI) inhibits the adipogenesis of porcine preadipocytes through Wnt/β-catenin signaling pathway. Biochem. Cell Biol. 2014;92:172–182. doi: 10.1139/bcb-2014-0011. [DOI] [PubMed] [Google Scholar]
- 6.Luo X., Hutley L.J., Webster J.A., Kim Y.H., Liu D.F., Newell F.S., Widberg C.H., Bachmann A., Turner N., Schmitz-Peiffer C., et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI) as a potent negative regulator of adipogenesis and modulator of autocrine/paracrine adipogenic factors. Diabetes. 2012;61:124–136. doi: 10.2337/db11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shangguan L., Ti X., Krause U., Hai B., Zhao Y., Yang Z., Liu F. Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells. 2012;30:2810–2819. doi: 10.1002/stem.1251. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z., Gao C., Ning Y., He X., Wu W., Chen Y.G. The pseudoreceptor BMP and activin membrane-bound inhibitor positively modulates Wnt/β-catenin signaling. J. Biol. Chem. 2008;283:33053–33058. doi: 10.1074/jbc.M804039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman E.D., Mattison G.L., Pira C.U., Oberg K.C. Defining the mechanism of limb regeneration: A potential novel role for BAMBI in mediating Fgf-induced Shh up-regulation; The FASEB Journal; In Proceedings of the Experimental Biology Meeting 2011; Washington, DC, USA. 9–13 April 2011; Bethesda, MD, USA: Federation of American Societies for Experimental Biology; 2011. [Google Scholar]
- 10.Brack A.S., Conboy I.M., Conboy M.J., Shen J., Rando T.A. A temporal switch from Notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Han X.H., Jin Y., Tan L., Kosciuk T., Lee J., Yoon J.K. Regulation of the Follistatin gene by RSPO-LGR4 signaling via activation of the Wnt/β-catenin pathway in skeletal myogenesis. Mol. Cell. Biol. 2014;34:752–764. doi: 10.1128/MCB.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X.H., Jin Y.R., Seto M., Yoon J.K. A WNT/β-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J. Biol. Chem. 2011;286:10649–10659. doi: 10.1074/jbc.M110.169391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochat A., Fernandez A., Vandromme M., Moles J.P., Bouschet T., Carnac G., Lamb N. Insulin and Wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol. Biol. Cell. 2004;15:4544–4555. doi: 10.1091/mbc.E03-11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y.J., Fang Y.H., Chi H.C., Chang L.C., Chung S.Y., Huang W.C., Wang X.W., Lee K.W., Chen S.L. Insulin and LiCl synergistically rescue myogenic differentiation of FoxO1 over-expressed myoblasts. PLoS ONE. 2014;9:e88450. doi: 10.1371/journal.pone.0088450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentzinger C.F., Wang Y.X., Rudnicki M.A. Building Muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Maltzahn J., Chang N.C., Bentzinger C.F., Rudnicki M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Amerongen R., Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 18.Ridgeway A.G., Petropoulos H., Wilton S., Skerjanc I.S. Wnt signaling regulates the function of MyoD and myogenin. J. Biol. Chem. 2000;275:32398–32405. doi: 10.1074/jbc.M004349200. [DOI] [PubMed] [Google Scholar]
- 19.Fujimaki S., Hidaka R., Asashima M., Takemasa T., Kuwabara T. Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running. J. Biol. Chem. 2014;289:7399–7412. doi: 10.1074/jbc.M113.539247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C.H., Neiswender H., Baik E.J., Xiong W.C., Mei L. β-catenin interacts with MyoD and regulates its transcription activity. Mol. Cell. Biol. 2008;28:2941–2951. doi: 10.1128/MCB.01682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai Y., Zhang Z., Dong P., Yang H., Yang G., Sun S. BAMBI inhibits porcine preadipocyte differentiation by facilitating ERK1/2 phosphorylation. Sheng Wu Gong Cheng Xue Bao. 2014;30:1531–1540. (In Chinese) [PubMed] [Google Scholar]
- 22.Jho E.H., Zhang T., Domon C., Joo C.K., Freund J.N., Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung J.Y. Activation of axin2 expression by β-catenin-T Cell Factor. A feedback repressor pathway regulating Wnt signaling. J. Biol. Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Shi S., Zhang J., Zhou F., Ten D.P. Wnt/β-catenin signaling changes C2C12 myoblast proliferation and differentiation by inducing Id3 expression. Biochem. Biophys. Res. Commun. 2012;419:83–88. doi: 10.1016/j.bbrc.2012.01.132. [DOI] [PubMed] [Google Scholar]
- 25.Klein P.S., Melton D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedgepeth C.M., Conrad L.J., Zhang J., Huang H., Lee V.M.Y., Klein P.S. Activation of the Wnt signaling pathway: A molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 27.Sekiya T., Adachi S., Kohu K., Yamada T., Higuchi O., Furukawa Y., Nakamura Y., Nakamura T., Tashiro K., Kuhara S., et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-β signaling, as a target of the β-catenin pathway in colorectal tumor cells. J. Biol. Chem. 2004;279:6840–6846. doi: 10.1074/jbc.M310876200. [DOI] [PubMed] [Google Scholar]
- 28.Huang J., Mo C., Romero-Suarez S., Bonewald L., Brotto M. Wnt3a potentiates myogenesis in C2C12 myoblasts by orchestrated changes in IP3-mediated Calcium signaling and β-catenin activation; Proceedings of Annual Meeting of the American-Society-for-Bone-and-Mineral-Research 2013; Baltimore, MD, USA. 4–7 October 2013; Hoboken, NJ, USA: Wiley-Blackwell; 2013. [Google Scholar]
- 29.Langen R., van der Velden J., Kelders M., Laeremans H., Schols A. Wnt3a promotes β-catenin signaling and myotube formation during myogenic differentiation. FASEB J. 2008;22:754.21. [Google Scholar]
- 30.Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]