Abstract
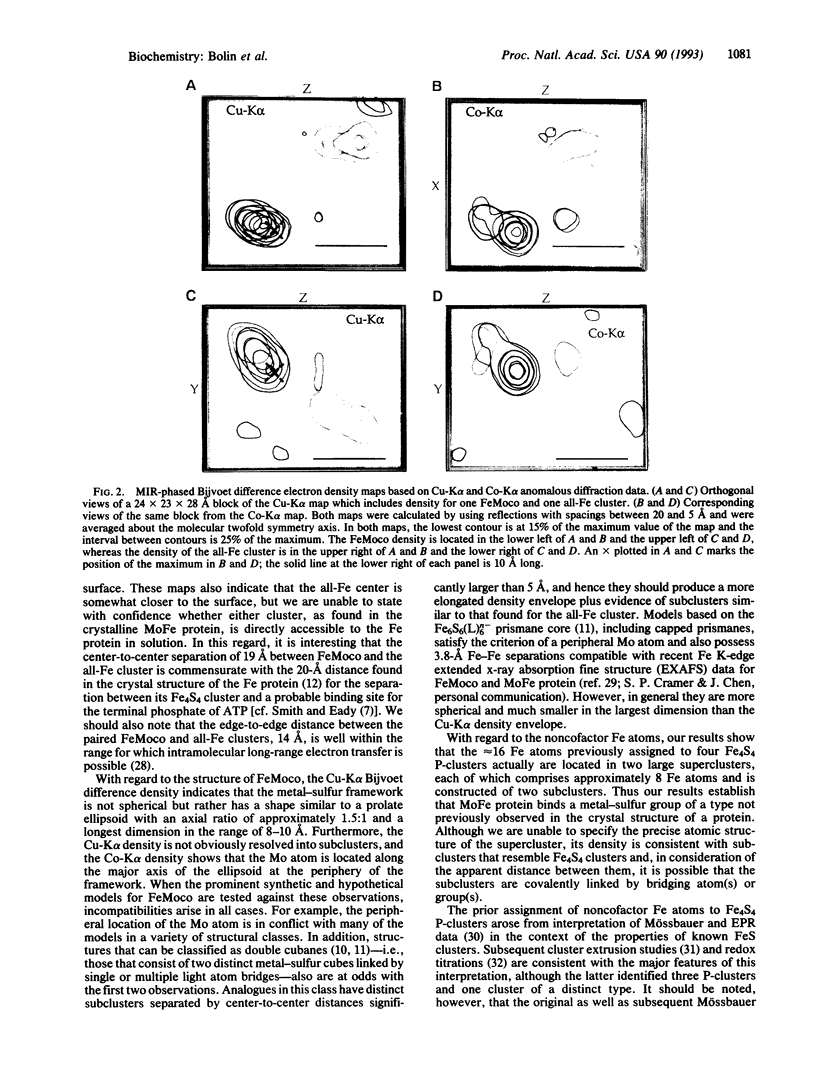
Nitrogenase (EC 1.18.6.1) catalyzes the conversion of dinitrogen to ammonia, the central reaction of biological nitrogen fixation. X-ray anomalous diffraction data were analyzed to probe the structures of the metal clusters bound by nitrogenase MoFe protein. In addition to one FeMo cofactor, each half-molecule of MoFe protein binds one large FeS cluster of a type not previously observed in a protein. The FeS cluster contains roughly eight Fe atoms, comprises two subclusters, and is separated from the FeMo cofactor by an edge-to-edge distance of 14 A. The inorganic framework of the FeMo cofactor is not resolved into subclusters, but the Mo atom is located at its periphery. FeMo cofactors in different half-molecules are 70 A apart and cannot promote binuclear activation of dinitrogen by two Mo atoms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber J. M., Flood A. C., Garner C. D., Gormal C. A., Hasnain S. S., Smith B. E. Iron K-edge X-ray absorption spectroscopy of the iron-molybdenum cofactor of nitrogenase from Klebsiella pneumoniae. Biochem J. 1988 Jun 1;252(2):421–425. doi: 10.1042/bj2520421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris R. H. Nitrogenases. J Biol Chem. 1991 May 25;266(15):9339–9342. [PubMed] [Google Scholar]
- Hagen W. R., Wassink H., Eady R. R., Smith B. E., Haaker H. Quantitative EPR of an S = 7/2 system in thionine-oxidized MoFe proteins of nitrogenase. A redefinition of the P-cluster concept. Eur J Biochem. 1987 Dec 15;169(3):457–465. doi: 10.1111/j.1432-1033.1987.tb13633.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991 Oct 4;254(5028):51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Smith J. L., Sheriff S. Direct phase determination based on anomalous scattering. Methods Enzymol. 1985;115:41–55. doi: 10.1016/0076-6879(85)15006-8. [DOI] [PubMed] [Google Scholar]
- Kurtz D. M., Jr, McMillan R. S., Burgess B. K., Mortenson L. E., Holm R. H. Identification of iron-sulfur centers in the iron-molybdenum proteins of nitrogenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4986–4989. doi: 10.1073/pnas.76.10.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P. A., Papaefthymiou V., Orme-Johnson W. H., Münck E. Mössbauer studies of solid thionin-oxidized MoFe protein of nitrogenase. J Biol Chem. 1988 Dec 25;263(36):19412–19418. [PubMed] [Google Scholar]
- McLean P. A., Papaefthymiou V., Orme-Johnson W. H., Münck E. Isotopic hybrids of nitrogenase. Mössbauer study of MoFe protein with selective 57Fe enrichment of the P-cluster. J Biol Chem. 1987 Sep 25;262(27):12900–12903. [PubMed] [Google Scholar]
- Meyer J., Zaccai G. Neutron small angle scattering of the Mo-Fe protein (nitrogenase) from Clostridium pasteurianum. Biochem Biophys Res Commun. 1981 Jan 15;98(1):43–50. doi: 10.1016/0006-291x(81)91867-2. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Seefeldt L. C., Morgan T. V., Dean D. R., Mortenson L. E. Mapping the site(s) of MgATP and MgADP interaction with the nitrogenase of Azotobacter vinelandii. Lysine 15 of the iron protein plays a major role in MgATP interaction. J Biol Chem. 1992 Apr 5;267(10):6680–6688. [PubMed] [Google Scholar]
- Smith B. E., Eady R. R. Metalloclusters of the nitrogenases. Eur J Biochem. 1992 Apr 1;205(1):1–15. doi: 10.1111/j.1432-1033.1992.tb16746.x. [DOI] [PubMed] [Google Scholar]
- Strahs G., Kraut J. Low-resolution electron-density and anomalous-scattering-density maps of Chromatium high-potential iron protein. J Mol Biol. 1968 Aug 14;35(3):503–512. doi: 10.1016/s0022-2836(68)80010-5. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Haaker H., Van Breemen J. F., Van Bruggen E. F., Eady R. R. Structure of the Mo-Fe protein component of Azotobacter vinelandii nitrogenase. Analytical ultracentrifugation and electron microscopy studies. Eur J Biochem. 1983 Nov 2;136(2):397–401. doi: 10.1111/j.1432-1033.1983.tb07755.x. [DOI] [PubMed] [Google Scholar]
- Weininger M. S., Mortenson L. E. Crystallographic properties of the MoFe proteins of nitrogenase from Clostridium pasteurianum and Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1982 Jan;79(2):378–380. doi: 10.1073/pnas.79.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Weininger M. S., Mortenson L. E., Rossmann M. G. Molecular symmetry of the MoFe protein of nitrogenase. Structural homology/nitrogen fixation/x-ray crystallography. J Biol Chem. 1982 Feb 10;257(3):1221–1223. [PubMed] [Google Scholar]
- Zimmermann R., Münck E., Brill W. J., Shah V. K., Henzl M. T., Rawlings J., Orme-Johnson W. H. Nitrogenase X: Mössbauer and EPR studies on reversibly oxidized MoFe protein from Azotobacter vinelandii OP. Nature of the iron centers. Biochim Biophys Acta. 1978 Dec 20;537(2):185–207. doi: 10.1016/0005-2795(78)90504-4. [DOI] [PubMed] [Google Scholar]