Fig. 1.
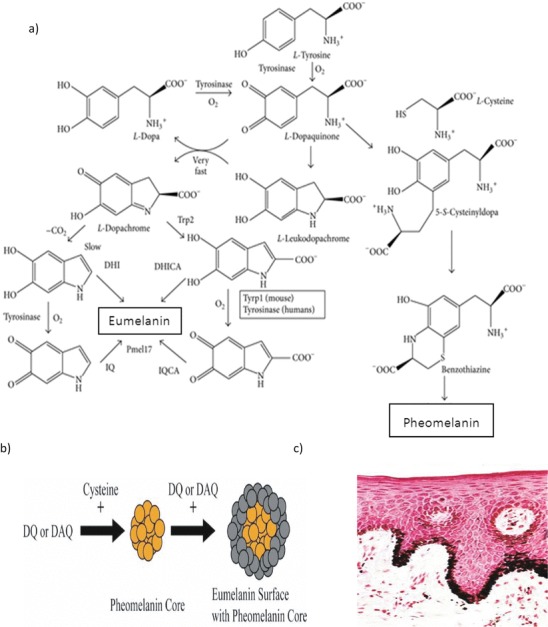
(a) Raper–Mason pathway for eumelanin and pheomelanin formation. The initial phase until l-dopaquinone is common to pheomelanin and eumelanin through the oxidation of l-tyrosine catalysed by the two activities of tyrosinase, tyrosine hydroxylase and dopa oxidase. l-Dopa quinone is the pivotal intermediate. Addition of l-cysteine gives place to benzothiazine units and then to pheomelanins (right side of the pathway). In the absence of l-cysteine, eumelanogenesis takes place since l-dopaquinone spontaneously cycled to l-leukodopachrome. This indoline reacts with l-dopaquinone in a very fast spontaneous reaction to yield l-dopachrome. l-Dopachrome is converted to DHI and DHICA mixtures, according to Trp2 activity and decarboxylation rate. Further oxidation of these dihydroxyindoles by tyrosinase or Trp1 gives place to indolequinones, and subsequent cross-link reactions between hydroxy and quinone forms lead to the polymer (Solano 2014). (b) Representation of eumelanin stacking on top of a core of pheomelanin. DQ, dopaquinone, and DAQ, dopamine quinone, from Ito and Wakamatsu (2008) and Sulzer and Zecca (1999). (c) Fontana–Masson stain shows melanin granules by reduction of silver nitrate to metallic silver at pH 4.0, shown as accumulation of black material in the cytoplasm of skin keratinocytes
