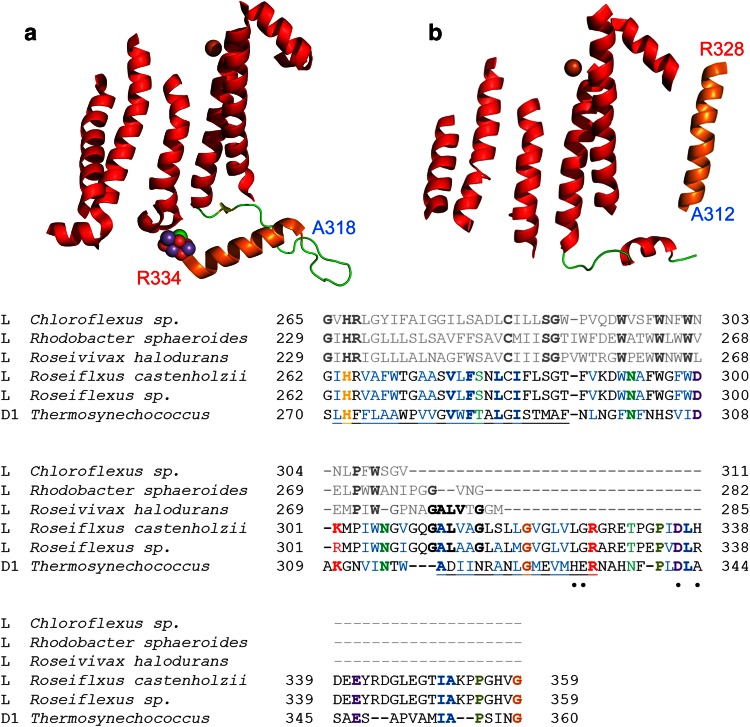
Fig. 6.
Sequence alignment of the L subunit from Roseiflexus spp. and the D1 subunit from T. elongatus. The alignment shows that the parallel alpha helix that in D1 is essential for the assembly and coordination of the Mn4CaO5 cluster a has sequence and structural homology to a putative sixth transmembrane helix predicted from the fused LM reaction center subunit in Roseiflexus (b). In bold colored letters, the conserved amino acids between the two Roseiflexus sequences and T. elongatus are highlighted. The colored letters that are not in bold show positive amino acid substitutions. The underline highlights the fifth transmembrane helix and the following alpha helix (parallel in D1 and transmembrane in L from Roseiflexus spp.). Sequences from a few phototrophic Chloroflexi and Proteobacteria strains are also shown. Surprisingly, the L subunit from the proteobacterium Roseivivax halodurans extends middle way through the predicted sixth helix. The black dots under the T. elongatus sequence are the ligands to the Mn4CaO5 cluster. a The transmembrane and parallel alpha helices of D1. b A homology model of the L subunit from Roseiflexus built using the subunit from B. viridis as a template. The model was made with the SWISS-MODEL automated service (Guex et al. 2009). The position of the putative sixth helix depicted in b is only hypothetical and just for illustration purposes